Graphical summary. Credit: Nucleic acid research (2024). DOI: 10.1093/nar/gkae776
A research team from the Institute of Neurosciences of the University of Barcelona (UBneuro) carried out a study describing a new molecular mechanism that affects RNA processing and modifies the process of protein synthesis in the brains of patients with of Alzheimer’s disease.
The study, which was carried out on post-mortem patient samples and animal models of the disease, will stimulate the design of future therapies to treat this dementia and other neurological disorders.
Cristina Malagelada, who led the study, and Genís Campoy-Campos, its first author, published the article in Nucleic acid research. Malagelada is a professor at the UB Faculty of Medicine and Health Sciences and at UBneuro and, together with Campoy-Campos, are members of the Center for Biomedical Research in Neurodegenerative Diseases (CIBERNED).
A new function for the RTP801 protein
Alzheimer’s disease is the most common type of dementia and causes progressive decline in cognitive abilities, memory and language, as well as emotional and psychiatric disorders. It is characterized by the accumulation of β-amyloid plaques outside neurons and hyperphosphorylated tau protein inside neurons, which impair brain function and cause cell death.
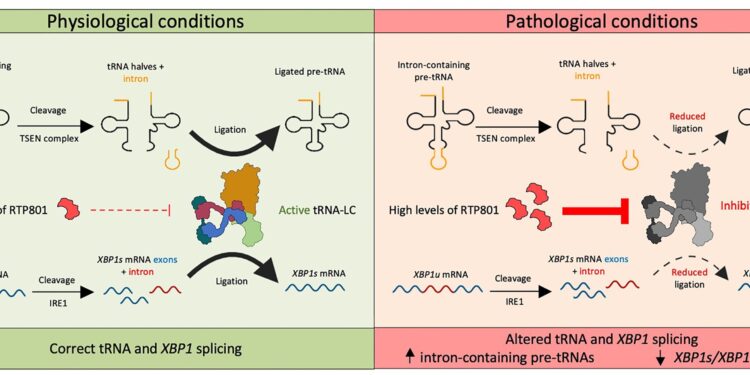
This study reveals a previously unknown role of the protein RTP801, a stress response factor abundant in patients with neurodegenerative diseases such as Alzheimer’s disease. According to the results, this protein can modify the molecular mechanisms that support neuronal survival by affecting the translation of RNA into proteins.
Malagelada says: “Until now, we knew that the RTP801 protein, present in hippocampal neurons, was involved in the pathology of Alzheimer’s disease, as we published in a previous article. At the time, we discovered that levels of this protein were significantly elevated. in mouse models of Alzheimer’s disease and in postmortem patient samples, and these values were correlated with disease progression.
“On a mechanistic level, we observed that reducing RTP801 expression prevented cognitive deficits and inflammation, notably by attenuating the activation of the hippocampal inflammasome, i.e. machinery that processes cytokines in inflammatory responses and results in gliosis (reactivation and proliferation of glial cells).” continues the expert.
Why is this mechanism crucial for neuronal health?
The study describes how the RTP801 factor negatively regulates the activity of the tRNA ligase complex (tRNA-LC), which is essential for the processing of RNA molecules. In the context of Alzheimer’s disease, higher levels of RTP801 may inhibit this complex and cause problems with RNA splicing and subsequent production of relevant proteins, such as brain-derived neurotrophic factor (BDNF). , exacerbating cognitive problems in a mouse model of Alzheimer’s disease.
Campoy-Campos notes that “in this study, we found that high levels of RTP801 interfere with the tRNA ligase complex, which is responsible for processing RNA, particularly in the process of ligation of its exons, a once the introns have been cleaved. The process takes place in both messenger RNA – which contains the information needed to build the protein – and transfer RNA, which carries the amino acids to translate it. »
The researcher emphasizes that “this process is vital for the correct synthesis of proteins at the level of the ribosome, the cellular organelle where the translation of RNA into proteins takes place.”
“Interestingly, this interaction between RTP801 and the tRNA ligase complex also affects the RNA binding of a transcription factor called XBP1. This factor helps cells deal with stress in the endoplasmic reticulum, an organ formed by a set of cisternae and membranous cavities in the cell and promotes the expression of BDNF, a neurotrophin crucial for synaptic transmission, memory and neuronal survival,” adds Campoy-Campos.
Impaired RNA processing, a consequence of high RTP801 levels, is extremely detrimental to neurons, disrupting their ability to synthesize proteins and respond to stress. As Malagelada points out, this change in RNA processing adds a new toxic component to the previously known course of Alzheimer’s disease.
“We are now addressing unbound RNA toxicity and its consequences as a new neurodegenerative mechanism in Alzheimer’s disease,” she says.
Boosting future therapies to treat neurodegenerative diseases
The discovery of new functions of the RTP801 protein could open future therapeutic options to address the treatment of neurodegenerative pathologies and preserve brain functions and neuronal health.
In this sense, Malagelada emphasizes that “if we manage to design inhibitors of the RTP801 protein – which we are currently working on – or to preserve the activity of the tRNA ligase complex, we could specifically block the most toxic functions of this factor and preserve essential functions. neural processes.
The researchers conclude that “this offers a new range of innovative therapeutic options in the context of these neurological disorders.”
More information:
Genís Campoy-Campos et al, RTP801 interacts with the tRNA ligase complex and deregulates its RNA ligase activity in Alzheimer’s disease, Nucleic acid research (2024). DOI: 10.1093/nar/gkae776
Provided by the University of Barcelona
Quote: Researchers identify molecular mechanism that could help design future therapies to treat Alzheimer’s disease (October 18, 2024) retrieved October 18, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.