Credit: Cell (2023). DOI: 10.1016/j.cell.2023.11.019
Researchers have discovered new disrupted genes and an unexpected molecular pattern — called BREACHes — linked to fragile woman in 11,000.
The study, by researchers at the University of Pennsylvania Perelman School of Medicine, which used brain cells and tissue donated by patients, also showed that simply changing the length of the abnormal repeating pattern could restore silenced genes on multiple chromosomes. The study was published in the journal Cell.
“Our findings have implications for future treatment strategies for fragile X syndrome and highlight potential mechanisms contributing to genome instability that may also underlie other diseases,” said Linda Zhou, MD, Ph.D., co-first author of the study, clinical resident in dermatology. at Penn Medicine.
A team led by lead author Jennifer Phillips-Cremins, Ph.D., associate professor of bioengineering and genetics and member of the Epigenetics Institute at Penn Medicine, studied FXS, the most common form of hereditary intellectual disability, in order to add an understanding of the underlying cause of the disorder. Hand models attribute it to the silencing of a single gene, FMR1, and the loss of the protein encoded by FMR1, Fragile X Messenger Ribonucleoprotein (FMRP).
It is widely believed that loss of FMRP causes severe dysregulation of synapses, which connect neurons in the brain, as well as disruption of the way genes are expressed in neuron nuclei. The main model of FXS was built on studies using a transgenic mouse in which the FMR1 gene was inactivated. However, the mouse model lacked the critical genetic driver of FXS: a mutation called “repeat expansion,” which occurs when a long repeat of a sequence of two or more DNA letters becomes unstable and abnormally long (a repeat of mutation length).
For FXS, this is the three-letter sequence – CGG – appearing at one end of the FMR1 gene. While a normal version of FMR1 has 40 or fewer CGG triplets in the repeating tract, an FXS patient will have 200 or more triplets. The abnormality triggers a defensive response from the cell, which essentially silences FMR1 and FMRP. Because the repetitive sequence is difficult to engineer, small animal models of FXS lack the repeat tract and therefore may not have demonstrated important aspects of the role of repetitive DNA in the mechanisms underlying the FXS.
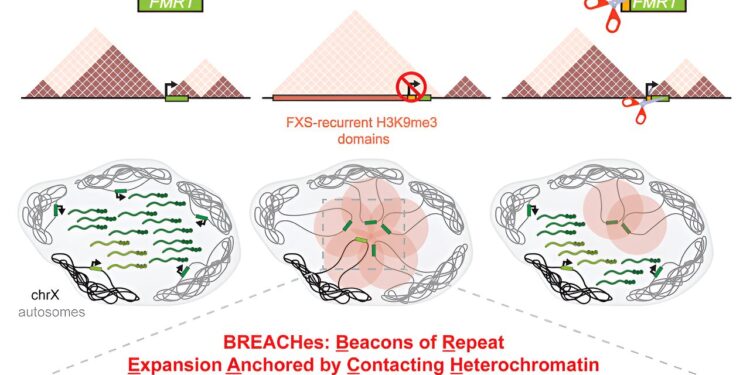
In their new study, the research team used a range of advanced sequencing and imaging techniques, as well as human cell lines and brain tissues with CGG repeat expansion, to discover surprising new patterns of disruption of the genome in FXS. The researchers found that large stretches of multiple chromosomes in FXS patient samples, which include the CGG repeat, are marked by inactivating heterochromatin. These heterochromatin “domains” are called BREACHes – Repeat Expansion Anchor Tags in Contact with Heterochromatin.
BREACHes cluster in physically contacting groups in the nucleus and silence genes involved in the synaptic connections of neurons, as well as genes related to the integrity of connective tissue such as skin and joints. Disturbances of synapses and connective tissue are observed in FXS patients in the clinic. Therefore, the ability to identify BREACHes has the potential to be a powerful tool for finding potentially important disrupted genes beyond FMR1.
The researchers also tested whether the repeat could be directly linked to BREACHes by using CRISPR-Cas gene editing technology to reduce the CGG expansion to a length not causing FXS.
“When we reduced CGG to a shorter length called a premutation (100 to 190 triplets), we observed that many of the large heterochromatin-inhibiting bands were inverted and that several chromosomes were spatially disconnected from FMR1,” he said. said co-lead authors Ken Chandradoss, Ph.D. and Ravi Boya, Ph.D., both postdoctoral researchers in the Phillips-Cremins lab.
The team’s experiments demonstrated that genes initially silenced by BREACHes were reexpressed in FXS cells with the CRISPR-shortened CGG repeat.
“The overall impact of our finding that mutation length CGG expansion is necessary for maintenance of BREACHes is that repeat engineering alone can potentially be used as a therapeutic approach to reverse genome-wide inactivation of several critical genes potentially contributing to clinical presentations of FXS,” said co-senior author Thomas Malachowski, a Ph.D. student in the Cremins laboratory.
Future FXS treatments could explore replacing the functions of some of the silenced genes identified in the study, not just FMR1. The researchers noted, however, that a more ambitious strategy would be to reduce the excessively long expansion of CGG repeats at a defined point in development to prevent or at least reverse the effects of heterochromatin silencing.
To explore this possibility, one would need to carefully balance the positive effects of reactivation of important genes with the protective role of heterochromatin in guarding against repetitive genome instability.
Other disorders potentially affected by these findings include Huntington’s disease and amyotrophic lateral sclerosis (Lou Gehrig’s disease), which are part of the same broader class of repeat expansion disorders as FXS, which is thought to they are due to the mutation of a single repetitive tract in the DNA.
Phillips-Cremins also explained that the team has observed VIOLATIONS in other human cellular models of genome instability, including with cell lines containing mutations found in cancer or in laboratory-induced DNA breaks.
“Our results suggest that BREACHes may in the future have a broader impact on gene silencing in other diseases with genome instability, including certain cancers and other repeat expansion disorders,” a- she declared.
More information:
Thomas Malachowski et al, Spatially coordinated heterochromatinization of long synaptic genes in fragile X syndrome, Cell (2023). DOI: 10.1016/j.cell.2023.11.019
Cell
Provided by the Perelman School of Medicine at the University of Pennsylvania
Quote: Researchers discover unexpected molecular pattern in fragile X syndrome (December 27, 2023) retrieved December 27, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.