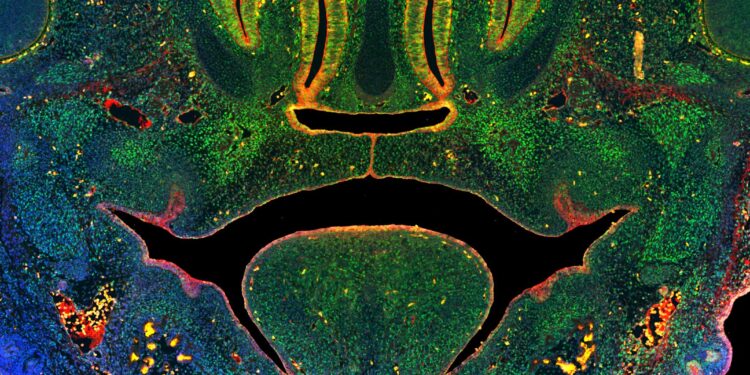
This image of a cross-section of the mid-face of a mouse embryo illustrates the fusion of tissues that form the secondary palate above the tongue. Green coloring illustrates cells expressing a key enzyme involved in DNA methylation, blue indicates nuclei of all cells, red indicates epithelial cells. Credit: University of Wisconsin-Madison
Cleft lip and palate are the most common craniofacial birth defects in humans, affecting more than 175,000 newborns worldwide each year. Yet despite decades of research, it is still unclear what causes most cases or what can be done to prevent them.
A recent study from the University of Wisconsin School of Veterinary Medicine (SVM) has uncovered new insights into orofacial development in mice that researchers believe could one day help reduce the risk of these malformations congenital in humans.
Published in the Proceedings of the National Academy of Sciences (PNAS), the study provides the first direct evidence of a mechanism called DNA methylation, which is necessary for craniofacial development. DNA methylation is a process by which a group of molecules are added to DNA to change gene expression without actually altering the DNA sequence. It is also affected by various environmental factors. Researchers found that disrupting DNA methylation interferes with the development of the lip and palate and causes these birth defects in mice.
Led by Robert Lipinski, associate professor of comparative biosciences at the UW School of Veterinary Medicine, the research is an important step toward developing preventative strategies that could one day reduce the risk of cleft lip and palate, known collectively as the name orofacial clefts (OFC), in animals and humans.
“We knew from previous research that genetics and environment interact to cause these types of birth defects, but our understanding of the environmental component lagged far behind that of genetics,” Lipinski says.
“Unlike genetics, we do not have a permanent record of the prenatal environment that can be examined retrospectively, but linking OFCs to DNA methylation helps us focus on the particular environmental influences that alter the risk of these types of birth defects.”
His team’s work has confirmed the essential role of DNA methylation in the regulation of orofacial development during embryonic development and demonstrates how disruptions of this process modify the ability of stem cells to form the connective tissue of craniofacial bone and cartilage, thus leading to the appearance of OFC.
Lipinski and his team achieved these results by genetically manipulating DNA methylation in two distinct groups of mouse embryos. The experiments yielded seemingly contradictory results, with OFCs growing in one group of mice but not the other.
To understand why there was a difference between the groups, the team conducted another series of experiments in which they inhibited DNA methylation in mouse embryos at different stages of development. The timing of DNA methylation was critical for the development of orofacial clefts.
They found that exposure on day 10 of gestation resulted in OFCs, but administration of the same inhibitor only 48 hours later resulted in normal orofacial development.
According to Lipinski, identifying this narrow window of gestational sensitivity is important because it not only helps target the next stage of his team’s research, but also helps design future public education initiatives once the we will better understand the modifiable environmental and behavioral risk. Factors that impact the risk of OFC in humans.
The 10th day of gestation in mouse embryos corresponds to the start of the 5th week of embryonic development in humans, a stage at which many pregnancies are not yet recognized.
“We know that DNA methylation can be influenced by a variety of environmental factors, including maternal stress, diet and exposure to drugs, toxins and environmental pollutants, and a better understanding of how the “Orofacial development is regulated by environmentally sensitive mechanisms that could directly inform birth defects. Prevention strategies,” he says.
“This next phase of our team’s research focuses on identifying specific factors that influence DNA methylation during orofacial development and therefore could modify OFC risk.”
Lipinski and his team are uniquely positioned to pursue this next stage of research because of another important result of the study: a new in vitro model developed by the team. The model will allow them to quickly examine thousands of dietary and environmental factors on a laboratory plate before testing the impact of specific factors on cleft susceptibility in mouse models.
The results obtained in cellular and animal models will help researchers identify more quickly and precisely the factors likely to have consequences on human development.
Orofacial clefts of the upper lip and palate affect approximately one in 700 newborns, and people with OFC experience feeding difficulties as infants that require multiple surgeries, dental procedures, and speech therapy for childhood and adolescence. Studies have shown higher mortality rates across all stages of life for people with OFC.
More information:
Caden M. Ulschmid et al, DNA methylation-mediated disruption of cranial neural crest proliferation and differentiation causes orofacial clefts in mice, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2317668121
Provided by University of Wisconsin-Madison
Quote: Researchers discover new clues about the cause of craniofacial birth defects (January 23, 2024) retrieved January 23, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.