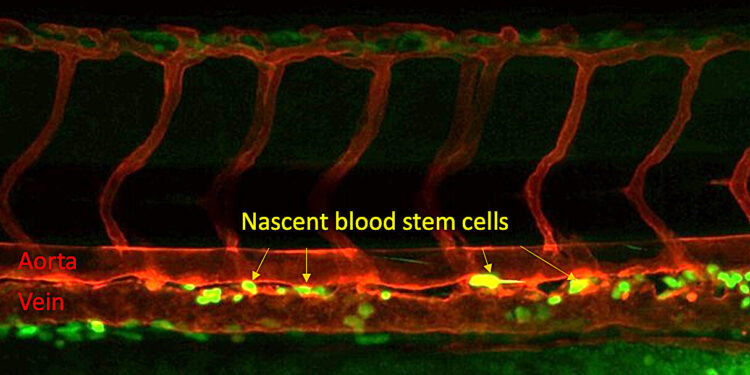
Blood stem cells form in the trunk of a zebrafish embryo. Blood stem cells are yellow, with the red tubes being the aorta at the top and a vein at the bottom. Credit: Xiaoyi Cheng.
A microbial sensor that helps identify and fight bacterial infections also plays a key role in the development of blood stem cells, providing valuable new insight into efforts to create patient-derived blood stem cells that could eliminate the need bone marrow transplants.
The discovery by a research team led by Raquel Espin Palazon, assistant professor of genetics, developmental and cell biology at Iowa State University, is published in Natural communications. It builds on previous work by Espin Palazon showing that the inflammatory signals that trigger a body’s immune response play an entirely different role in the early stages of life, as vascular systems and blood form. in embryos.
Espin Palazon said knowing that embryos activate the microbial sensor, a protein known as Nod1, to force vascular endothelial cells to become blood stem cells could help develop a method to make stem cells. laboratory tests using a patient’s own blood.
“This would eliminate the difficult task of finding compatible bone marrow transplant donors and the complications that arise after receiving a transplant, thereby improving the lives of many patients with leukemia, lymphoma and anemia,” a- she declared.
A critical signal
Stem cells are both the factories and raw materials of a body, dividing repeatedly to self-renew and build new cells for specific tissues. Pluripotent stem cells in embryos can produce any type of cell the body needs, while adult stem cells are limited to producing particular types. Blood stem cells, or hematopoietic stem cells, make up all the components of blood. A lifetime supply of blood stem cells is created before birth inside an embryo.
The immune receptor identified by Espin Palazon’s team activates in an embryo before endothelial cells begin to become stem cells, preparing them for the transition.
“We know that blood stem cells form from endothelial cells, but the factors that caused the cell to change its identity were enigmatic,” she said. “We didn’t know that this receptor was necessary or that it was needed so early, even before blood stem cells formed.”
The researchers focused on Nod1 by analyzing public databases of human embryos and studied it using zebrafish, which shares about 70% of its genome with humans. The creation of blood stem cells closely tracked Nod1 levels, as its effects were inhibited or enhanced.
To confirm that Nod1 also plays a role in human blood development, the research team worked with Children’s Hospital of Philadelphia. Researchers there produce human-induced pluripotent stem cells, which are generated from mature samples but genetically reprogrammed to behave like the versatile stem cells found in embryos.
Induced pluripotent stem cells can create most types of blood cells, but not functional blood stem cells. But when the researchers removed Nod1, blood production faltered, as was the case with zebrafish blood stem cells.
In search of self-derived stem cells
Understanding that Nod1 is a prerequisite for the development of blood stem cells is progress for scientists who hope to design a system for producing blood stem cells from human samples, which could offer a revolutionary new option for patients suffering from blood disorders . Instead of a life-saving infusion of blood stem cells via a bone marrow transplant, the spongy interior of bones that contains most of the body’s blood stem cells, patients could be treated with stem cells from their own bodies.
Self-derived stem cells could avoid the risks of graft-versus-host disease, a common and potentially fatal reaction that occurs when a patient’s immune system perceives the transplant as a threat of attack.
“This would be a huge advancement for regenerative medicine,” Espin Palazon said.
Espin Palazon’s team continues to unravel the complex interactions in which blood stem cells appear, notably by refining the timeline. Understanding when signals are expressed is essential to developing methods for making blood stem cells.
“Timing is crucial. It’s like when you’re cooking and you have to add the ingredients in a specific order,” she said.
Further research will benefit from collaboration with Children’s Hospital of Philadelphia, which trained one of the study’s co-authors, Clyde Campbell, adjunct assistant professor of genetics, developmental and cell biology, in protocols allowing the creation of induced pluripotent stem cells.
“My group at Iowa State University will continue to work toward a life free of blood disorders. I believe our research will pave the way to finally creating therapeutic-grade blood stem cells to cure patients with blood disorders ” said Espin Palazon.
More information:
Xiaoyi Cheng et al, Nod1-dependent NF-kB activation initiates hematopoietic stem cell specification in response to small Rho GTPases, Natural communications (2023). DOI: 10.1038/s41467-023-43349-1
Provided by Iowa State University
Quote: Researchers discover crucial step in creating blood stem cells (December 19, 2023) retrieved December 19, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.