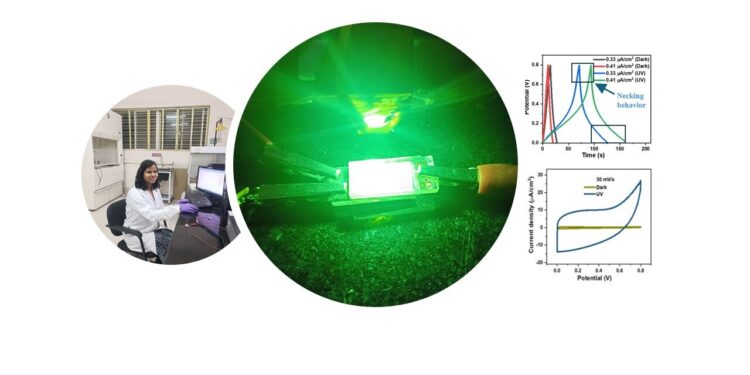
A photo-chargeable supercapacitor showed a 3,000% increase in capacitance under light compared to darkness. A novel shrinking behavior was discovered upon illumination. Credit: Santilata Sahoo
Researchers from the Department of Instrumentation and Applied Physics (IAP) at the Indian Institute of Science (IISc) and their collaborators have designed a novel supercapacitor that can be charged by light shining on it. Such supercapacitors can be used in various devices, including street lights and self-powered electronics such as sensors.
Capacitors are electrostatic devices that store energy as charges on two metal plates called electrodes. Supercapacitors are improved versions of capacitors: they exploit electrochemical phenomena to store more energy, explains Abha Misra, professor at IAP and corresponding author of the study published in the Journal of Materials Chemistry A.
The electrodes of the new supercapacitor are made of zinc oxide (ZnO) nanorods grown directly on fluorine-doped tin oxide (FTO), which is transparent. It was synthesized by Pankaj Singh Chauhan, first author and CV Raman postdoctoral researcher in Misra’s group at IISc.
Both ZnO and FTO are semiconductors with appropriately aligned energy levels, which enables superior performance of the photo-rechargeable supercapacitor. FTO, being transparent, allows light to fall on the optically active ZnO nanorods, which charges the supercapacitor. Chauhan explains that two electrolytes, a liquid and a semi-solid gel, were used as the conductive medium between the electrodes.
The charge storage capacity (capacitance) is inversely proportional to the distance between the electrodes.
“As the distance gets smaller, the capacitance increases,” Misra explains. In electrostatic capacitors, it is difficult to maintain a small distance between the electrodes. However, in a supercapacitor, the charges on the electrodes attract oppositely charged ions in the electrolyte, resulting in the formation of a layer of charge a few atoms apart, called an electric double layer or EDL. This results in the high capacitance of supercapacitors.
When the researchers exposed their supercapacitor to ultraviolet (UV) light, they saw a significant increase in capacitance, several times greater than previously described supercapacitors. They also noticed two unusual properties. First, while capacitance typically decreases as voltage increases, they found the opposite: The capacitance of their supercapacitor under UV light actually increased as voltage increased.
“We call this the necking phenomenon,” says AM Rao, a professor at Clemson University in the US and co-author. He explains that this may be due to the high porosity of the electrodes. Second, the energy stored in the supercapacitor typically decreases when it is charged more quickly, because the ions in the electrolyte do not move fast enough to keep up with the increased charging rate. However, with the liquid electrolyte, the team found that the energy stored in the supercapacitor surprisingly increased when it was charged rapidly under UV light.
Mihir Parekh, a postdoctoral researcher in Rao’s group, has developed theoretical models to explain these novel observations. These results pave the way for the development of fast-charging, high-energy-density supercapacitors, he suggests.
To design their current supercapacitor, the team explored two key ideas. First, the surface area of the electrodes was increased by combining two optically active semiconductor interfaces in a way that maximized interaction with light, leading to higher charge generation. Second, a liquid electrolyte was used to ensure efficient EDL. Together, these two elements resulted in superior performance.
“The ideas were simple… but when combined, they worked very well,” Misra says. She adds that refining the supercapacitor design can also enable it to be charged with visible and infrared light. The IISc-Clemson team aims to further explore and better understand the new phenomena observed in order to design better supercapacitors.
“Supercapacitors have many applications,” Misra says. For example, they can replace the solar cells used in streetlights. They have a high power density, which allows them to release charge faster than batteries. They can also be used to power chips in electronic devices such as mobile phones.
“We have miniaturized the supercapacitors to the micron scale so that they can be integrated into these microelectronic chips,” adds Misra.
More information:
Pankaj Singh Chauhan et al., Influence of electrolyte on the photo-charging capacity of a ZnO–FTO supercapacitor, Journal of Materials Chemistry A (2024). DOI: 10.1039/D4TA04702H
Provided by Indian Institute of Science
Quote: Researchers develop light-charged supercapacitor for self-powered devices (2024, September 5) retrieved September 5, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.