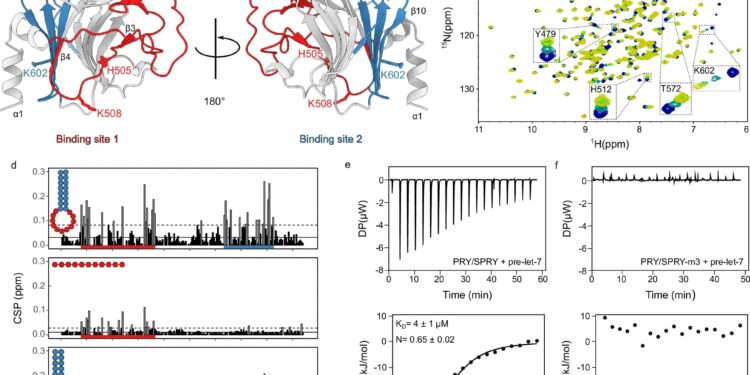
NMR and ITC analysis of RNA binding by the TRIM25 PRY/SPRY domain. Credit: Natural communications (2024). DOI: 10.1038/s41467-024-52918-x
An international research team led by Professor Janosch Hennig from the University of Bayreuth has discovered how the TRIM25 protein contributes to defense against RNA viruses whose genetic material is contained in the form of ribonucleic acid (RNA).
The results provide a better understanding of the molecular mechanisms of the human immune system. The researchers have now published their results in Natural communications.
The coronavirus has shown that there is a risk of a pandemic in the event of a mutation of viruses dangerous to humans: these mutations spread more quickly and are more difficult for the human immune system to combat. It is therefore all the more important to understand the molecular mechanisms of the proteins responsible for the innate immune response in humans. The new findings can then be used to develop new antiviral drugs to contain pandemics.
The TRIM25 protein plays a central role in innate immune defense against RNA viruses, but its role is still poorly understood. It is clear that TRIM25, as a so-called ubiquitin E3 ligase, triggers the immune system’s response to viral RNA by transferring the ubiquitin molecule to the RIG-I protein, which then activates the immune defense.
It was also discovered some time ago that TRIM25 itself could bind various forms of RNA. However, it was unclear how TRIM25 binds RNA and how this binding influences antiviral activity.
To better understand the underlying molecular mechanism, the research team led by Professor Hennig (Chair of Biochemistry IV) at the University of Bayreuth studied TRIM25-RNA binding in more detail.
Nuclear magnetic resonance (NMR) spectroscopy was carried out in Bayreuth and can be used to clarify the electronic environment of atoms and the interaction with neighboring atoms. Using this and other biophysical methods, the researchers identified the RNA-binding mechanism of TRIM25. Additionally, viral RNA sequences and structures to which TRIM25 specifically binds were identified.
In a next step, the scientists produced a TRIM25 mutant that cannot bind RNA. The researchers used this mutant to test the influence of RNA binding on the antiviral properties of TRIM25: they infected cell cultures lacking TRIM25 with a virus, then added regular TRIM25 or the mutant without binding capacity to RNA.
Examination of cultures showed that viral gene activity is significantly increased when TRIM25 cannot bind RNA. This indicates an essential role for TRIM25 RNA binding in antiviral activity.
More information:
Lucía Álvarez et al, Molecular dissection of the RNA binding mechanism of TRIM25 provides key insights into its antiviral activity, Natural communications (2024). DOI: 10.1038/s41467-024-52918-x
Provided by the University of Bayreuth
Quote: Researchers determine how a protein contributes to human immune defense against RNA viruses (October 2, 2024) retrieved October 2, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.