Graphical summary. Credit: Bioorganic chemistry (2024). DOI: 10.1016/j.bioorg.2024.107595
A study carried out by researchers from the Institute of Biomedicine of the University of Barcelona (IBUB) has developed a new pharmacological tool capable of simultaneously administering three oligonucleotide-based drugs, each acting against a different therapeutic target within the cell.
The article, published in the journal Bioorganic chemistrypresents proof of concept of this innovative triple-targeting compound, which has been successfully applied to breast cancer cells. According to the researchers, it is a therapy with “high potential for the treatment of complex diseases such as cancer or diabetes”.
The study is led by Professor Montserrat Terrazas, from the Department of Inorganic and Organic Chemistry of the Faculty of Chemistry of the UB, and by Sandra Pérez-Torras, lecturer in the Department of Biochemistry and Molecular Biomedicine of the Faculty of biology from the UB and researcher at the Network of the Biomedical Research Center on Liver and Digestive Diseases (CIBEREHD) and Sant Joan de Déu Research Institute (IRSJD).
Professor Marçal Pastor Anglada also participated and postdoctoral researcher Aida Mata-Ventosa and predoctoral researcher Ariadna Vila-Planas participated as first authors of the scientific article.
New strategy against drug resistance
Multifactorial or complex pathologies, such as cancer, are the result of the joint action of multiple factors in the body, which activate different tumor signaling pathways. This multiplicity of signals makes cancer cells resistant to certain treatments, because when the drug blocks the primary signaling pathway, the tumor’s secondary signaling pathways are activated.
“Small molecule drug combinations are often used to address these problems, but many of the combination therapies approved so far have several limitations, such as difficulty interacting with target proteins with poorly defined binding sets or toxicity problems due to the interaction between drugs of different chemical nature”, explain the researchers.
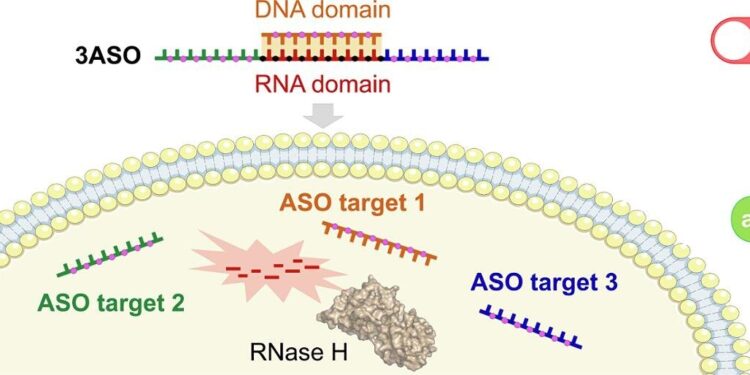
Faced with this challenge, the UB team developed a pharmacological tool that manages three antisense oligonucleotide (ASO) drugs. These are short chains of nucleic acids designed to bind to specific messenger RNAs and thus block the key function of this molecule in protein synthesis in cells.
This triple drug compound was successfully applied in breast cancer cells to inhibit the expression of three specific proteins (Akt, Hsp27 and HER2), which contribute to poor breast cancer prognosis and drug resistance.
The results of the new study show that the toxicity of this innovative therapy on cancer cells is greater than the combination of the three ASO-type drugs administered independently.
“In addition, the specificity of the chosen targets makes it possible to attack tumor cells presenting these alterations without affecting non-tumor cells,” add the researchers.
Activation of the new combination drug inside the cell is mediated by the enzyme RNase H, which is naturally found in the cell cytoplasm. “This enzyme recognizes the central part of the construct (a hybrid of RNA and DNA) and cleaves its RNA component, which acts as a connecting link between the three drugs. As a result, all three ASOs are released simultaneously and can exert their inhibitory functions,” the researchers explain.
The proof of concept was performed on cells from a specific type of breast cancer, but the experts note that the new tool could be used in the future to “deliver multiple combinations of ASOs that could be directed to multiple combinations of therapeutic targets not only for different types of cancer, but also applicable to other complex diseases, such as diabetes,” they conclude.
More information:
Aida Mata-Ventosa et al, Multifunctional constructs based on RNase H-sensitive ASOs as promising tools for the treatment of complex multifactorial pathologies, Bioorganic chemistry (2024). DOI: 10.1016/j.bioorg.2024.107595
Provided by the University of Barcelona
Quote: Researchers design a drug capable of acting simultaneously against three different therapeutic targets (October 8, 2024) retrieved October 8, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.