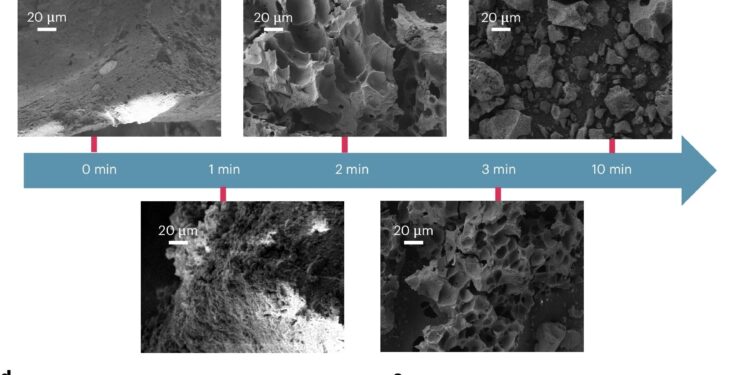
Thermal performance, product distribution and morphological evolution in microwave pyrolysis of EOL tires. Credit: Natural chemical engineering (2024). DOI: 10.1038/s44286-024-00110-9
Tires are an essential part of everyday life. Without them, our vehicles would just be a collection of pieces assembled, convenient to sit in, but not efficient to get where you’re going.
Although their usefulness is undeniable, tires nevertheless pose certain problems. A 2016 Federal Highway Administration report found that 280 million tires are thrown away each year in the United States. Globally, that number is much higher: more than 1 billion, according to a report from the World Business Council for Sustainable Development.
What to do with these used tires is a constant and often complicated conversation.
Tires are composite materials that contain many components, including a molecule known as 6PPD, which provides UV protection to help the rubber in tires last longer. 6PPD achieves this by absorbing the sun’s rays and preventing the material from breaking down due to reactions with ozone and other reactive oxygen species in the air.
However, as tires wear on road surfaces, they release 6PPD particles into the environment. Stormwater runoff carries these toxic particles into freshwater systems and other bodies of water, where the chemical can quickly kill fish, even in small doses. Recently, tribes in the Pacific Northwest filed a petition asking the Environmental Protection Agency to consider establishing regulations banning the use of the chemical.
University of Delaware researchers from the Center for Plastics Innovation and the Department of Chemical and Biomolecular Engineering, led by Dion Vlachos, Unidel Dan Rich Chair in Energy, have developed a method to address tire decontamination at the end of the 6PPD’s life.
The researchers recently published their approach in Natural chemical engineeringdemonstrating a way to turn 6PPD into safe chemicals and turn crumbled rubber scraps into aromatics and carbon black, a soot-like material found in everything from pigments to cosmetics to leather. ‘electronic.
Reuse tire materials safely
According to Vlachos, tires are responsible for about a third of microplastics in the environment. In fact, almost 25% of a tire’s components are made of synthetic rubber, which is a plastic.
Under exposure to solar radiation, 6PPD transforms into 6PPD-quinone, a so-called diketone, or a molecule composed of two ketone groups. Tires themselves are a major source of these diketone molecules. And it’s not just microplastics that result from tire wear during use. These molecules can also be released into the environment from tires left in landfills and exposed to the elements, such as precipitation.
“You can’t put a filter on the environment like you might have a filter on your home clothes dryer to capture these fibers,” said Vlachos, who also directs the Delaware Energy Institute.
While others in the field have attempted to break down tire materials using high heat, through a process known as pyrolysis, 6PPD is tenacious and the diketone molecules remain in the oil left behind. . If the oil is used in fuel or other materials, diketone molecules come with them, causing a problem.
So the Vlachos team decided to try to remove 6PPD via a process known as chemical extraction. This involved placing millimeter-sized pieces of tire, or rubber crumbs, into a conventional microwave reactor, heating the materials, and using a chemical solvent to quickly separate the 6PPD from the other molecules present.
Discover the latest in science, technology and space with more than 100,000 subscribers who rely on Phys.org for daily information. Sign up for our free newsletter and receive updates on the breakthroughs, innovations and research that matter:daily or weekly.
Once 6PPD molecules are removed, they can be chemically transformed into safe chemicals that can be used or sold inexpensively. The rest of the tire, meanwhile, can be recycled using conventional plastic recycling methods – a plus, given that there are currently no alternatives to tires in general. This would enable the restored tire materials to be used worry-free in practical applications, for example on football fields, playgrounds or in road asphalt.
Rubber crumbs could also be used in aromatics, which are raw materials for a wide range of consumer products, or as carbon black, a soot-like material found in many pigments, conductive/insulating elements and agents. reinforcement, among others.
The UD research team protected this new approach through the university’s Office of Economic Innovation and Partnerships.
To date, the research team has proven this approach on a laboratory scale, according to Vlachos, and a techno-economic analysis showed that the cost seems very reasonable. This is a positive step, but there is still work to be done and time is running out.
Worldwide, the number of end-of-life tires continues to rise, with some reports estimating that there could be as many as five billion tires due for disposal worldwide by 2030. At the same time, the use of used tires in the United States decreased by 25% between 2013 and 2021.
“I think recycling the tire itself is important, so there are truly circular solutions that do upcycling,” he said. “We need to make things at a large enough scale and at a reasonable cost outside the lab. This needs to be demonstrated with pilot plants. We haven’t done that.”
Moving solutions from the lab to the real world will require more engineering effort and time. Having a center dedicated to plastics innovation at UD is a definite advantage, Vlachos said, because it brings together a critical mass of people who talk, think and work on these issues. Startups and other minds, as well as the automotive industry, will play a key role in adopting solutions.
“We need to educate the community. We need social sensitivity, awareness. This is not a problem that will solve itself,” Vlachos said.
More information:
Sean Najmi et al, Decontamination of end-of-life tires from 6PPD and upcycling, Natural chemical engineering (2024). DOI: 10.1038/s44286-024-00110-9
Provided by University of Delaware
Quote: Decontamination of toxic tires: researchers demonstrate their ability to eliminate toxic particles from end-of-life tires (November 20, 2024) retrieved November 20, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.