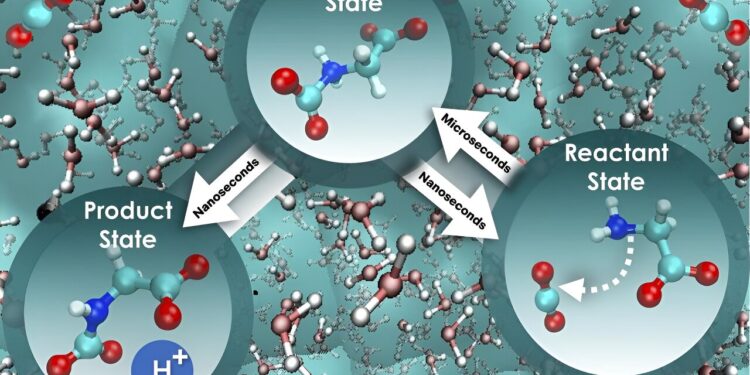
(Right to left) Carbon capture by aqueous glycine: the attack of the amino acid on carbon dioxide (reactive state) is strongly influenced by water dynamics, leading to a slow transition to a state intermediate. In the next step, due to the reduction of non-equilibrium solvent effects, a proton is rapidly released, leading to the product state. Credit: Santanu Roy/ORNL, US Department of Energy
Scientists at the Department of Energy’s Oak Ridge National Laboratory have taken a major step toward understanding a viable process for direct air capture, or DAC, of carbon dioxide from the atmosphere. This DAC process is in its early stages with the goal of achieving negative emissions, where the amount of carbon dioxide removed from the gas envelope surrounding the Earth exceeds the amount emitted.
The research, recently published in Cellular Reports Physical Sciences, focused on the fundamental steps of carbon dioxide sequestration using aqueous glycine, an amino acid known for its absorptive qualities. By combining a series of advanced computational methods, the scientists studied less explored dynamic phenomena in liquid solutions, related to the rate at which carbon dioxide can be captured.
“Chemical reactions in water are complicated, especially when the movement of water molecules plays an important role,” said Santanu Roy, who designed the computational investigation with his colleague Vyacheslav Bryantsev. “Water molecules and chemicals engage in something similar to a coupled dance that can slow the reaction slightly or significantly. Understanding these dynamic interactions, known as non-equilibrium solvent effects, is essential to get a complete picture of how reactions work and how quickly they occur.”
The researchers found that when looking at the rate at which carbon dioxide is absorbed, focusing only on the free energy barrier – the energy threshold that must be overcome for a system to move from one state to another – is an oversimplification that does not provide the completeness of the information. picture. This incomplete approach can lead to an inaccurate understanding of reaction kinetics, that is, the factors that influence the rate at which a reaction occurs.
“We used a more comprehensive approach that takes into account the influence of water on movement along the reaction path, and the result was intriguing,” Bryantsev said. “The initial step, where glycine interacts with carbon dioxide, is nearly 800 times slower than the next step, where a proton is released to ultimately form a mixture of products to retain the absorbed carbon dioxide.
“Surprisingly, the free energy barrier remains constant for both stages, and so this different perspective truly distinguishes the speed of these two critical stages and provides a pathway to increase the efficiency of carbon dioxide absorption and separation. carbon.”
The extensive ab initio molecular dynamics simulations used in this study were still limited by their short time and length scales and the high computational costs of representing chemical reactions.
“For future projects, we intend to combine the emerging machine learning approach with highly accurate simulations and develop interatomic interaction potentials based on deep neural networks. This will allow us to perform simulations molecular systems with high precision at large scales with significantly reduced computational costs,” said Xinyou Ma, who carried out the simulations.
Roy added: “Although we have built a molecular-level kinetic picture of carbon dioxide capture by aqueous amino acids, access to large length and time scales through the use of the approach machine learning will help us understand the effects of macroscopic factors such as temperature. , pressure and viscosity on the DAC and how these effects relate to the molecular image obtained.
Overall, the study results shed light on the complex functioning of DAC and highlight the critical role of kinetics, thermodynamics, and molecular interactions in the removal of carbon dioxide from the atmosphere by acids. aqueous amines. As these mechanisms become better understood, the prospect of deploying DAC technology on a large scale will become more feasible. Around the world, several different DAC projects are in various stages of research, testing and development.
More information:
Xinyou Ma et al, An ab initio free energy study of the reaction mechanism and rate-limiting steps of CO2 capture by aqueous glycine, Cellular Reports Physical Sciences (2023). DOI: 10.1016/j.xcrp.2023.101642
Provided by Oak Ridge National Laboratory
Quote: Researchers decode the potential of aqueous amino acids for direct capture of CO₂ in the air (December 1, 2023), retrieved December 2, 2023 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.