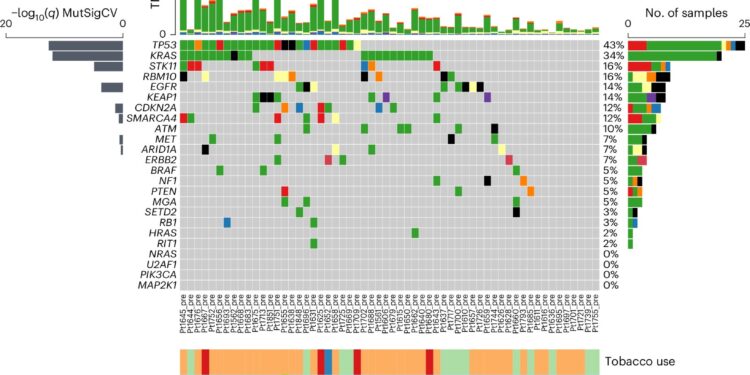
Neoantigen screening design and genomic characteristics of the NSCLC cohort. AWES was carried out on n = 58 pre-therapeutic samples and n = 42 samples being processed, including n = 36 cases with matched data before/under treatment. After prediction of neoantigens n = 14 patients underwent immunogenicity screening and subsequent data were used to model immunogenicity-determining features. bPre-therapy mutational landscape of common somatic mutations in NSCLC (n = 58). Top 3 mutational signature scores by DeconstructSig (SBS1, SBS4, SBS87). Frequency of clonal/subclonal variants (defined as ≥0.95 CCF blue bar or <0.95 CCF red bar). Change in copy number of fractions per samples represented as a bar graph (lower panel). delete, deletion; ins, insertion; NSQ, non-scaly; SQ, scaly. Credit: Natural medicine (2024). DOI: 10.1038/s41591-024-03240-y
A Cleveland Clinic-led research collaboration between Timothy Chan, MD, Ph.D., chairman of the Cleveland Clinic Global Center for Immunotherapy, and Bristol Myers Squibb has released the most comprehensive overview to date of how whose immune system remodels tumor architecture in response to immune checkpoint therapy.
The eight-year study, published in Natural medicinedescribes how cancer immunotherapy induces tumor recognition via neoantigens to remodel the tumor ecosystem. Neoantigens are small peptides produced when cancer cells mutate and are a primary marker for the immune system to recognize cancer cells as different from its own.
“This study is unique in that we sampled tumors before treatment and then shortly after initiation of immunotherapy,” says Dr. Chan, who is also chair of the Center for Immunotherapy & Precision Immuno-Oncology at Cleveland. Clinic, program manager of the Case Comprehensive Cancer Center. Immuno-Oncology Program and Sheikha Fatima bint Mubarak Endowed Chair in Immunotherapy. “Our goal was to understand how patients’ tumors are recognized and modified by their immune systems in response to immunotherapy.”
Our immune cells and cancer cells constantly interact and influence each other during cancer. Immunotherapy treatments must work in this context by strengthening our immune cells to eliminate cancer. Scientists like Dr. Chan have begun to unravel the complex relationships between treatment, immunity and cancer over the past 15 years, but human data are scarce.
The CheckMate-153 trial was overseen by pharmaceutical company Bristol Myers Squibb and Dr Chan’s team provided a central site for analysis of the trial. As part of the main trial, investigators included a biomarker substudy to identify how neoantigens determine response to nivolumab by sampling patients’ tumors before treatment and 3 weeks after treatment. From these tumor samples, sequencing was used to identify mutations creating neoantigens.
Neoantigens are thought to be the primary means by which the immune system recognizes tumors, but neoantigen prediction tools lack accuracy due to the lack of existing data in this area. To overcome this problem, the team developed the largest neoantigen screen to date, where they validated their predictions and monitored the dynamic response to neoantigens with longitudinal blood draws.
Within three weeks of treatment, people who responded well to nivolumab had a sharp decline in clonal neoantigens. Meanwhile, individuals whose cancer did not go into remission still developed an immunological response, but in smaller subclonal populations. This is important because many thought that non-responders were unable to activate and recognize the tumor, but here they show that it is possible for the immune system to mount a response to neoantigens, but this is insufficient to destroy all tumor clones.
Current neoantigen prediction tools rely heavily on HLA-binding neoantigens, but they lack the T-cell recognition aspect of immunogenicity, says Cleveland Clinic co-first author Tyler Alban, Ph. .D., project staff at Chan Lab. Dr. Alban, data scientist Prerana Parthasarathy and other team members developed a machine learning program that uses the new screening data to better predict immunogenic neoantigens. In doing so, the program identified novel features harbored by these cancer-derived neoantigens.
“We observed an entire ecosystem of immune cells at work, with each T cell recognizing a different neoantigen changing the clonal composition of the tumor,” explains Dr. Alban. “Our data allows us to generate new information on neoantigens and resistance to immunotherapy.”
By cataloging changes to neoantigens during treatment, Dr. Alban’s analyzes challenged the prevailing theory in immunotherapy: that a tumor only needs one lucky mutation to develop characteristics that our immune system recognizes. like a threat. The results show that many different T cells recognizing many different cancer characteristics are needed to respond well to treatment.
The roadmaps generated by these types of observational studies will be critical to future navigation of immuno-oncology research, says Dr. Chan.
“Finding out why our immune system responds to certain cancer mutations but not others is like the Holy Grail for immunotherapy researchers,” he explains. “Our findings are one of the closest things we have to understanding these things.”
The group is also using its dataset in collaboration with IBM in the Cleveland Clinic – IBM Discovery Accelerator to develop more advanced AI models that predict new molecules for cancer treatments and cancer vaccine development.
More information:
Tyler J. Alban et al, Neoantigen immunogenicity landscapes and evolution of tumor ecosystems during nivolumab immunotherapy, Natural medicine (2024). DOI: 10.1038/s41591-024-03240-y
Provided by Cleveland Clinic
Quote: Researchers build first large-scale atlas of how immune cells respond to mutations during cancer immunotherapy (October 1, 2024) retrieved October 1, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.