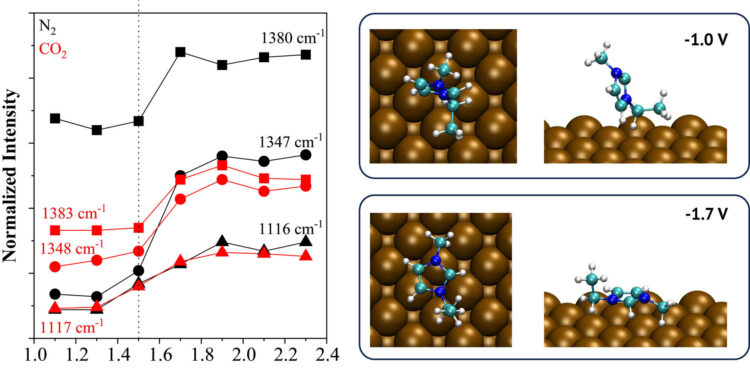
(A) Maximum SERS intensity as a function of applied absolute potential (|E-IRΩ|). The dotted line marks the potential for orientation change of imidazolium species under N2 (black) and CO2 (red). Summits: 1116 cm−1 (pentagons) for δ(C4VS5-H); 1347cm−1 for υ(I am ring)+υ(CH2(N)) (spheres); 1380cm−1 for υ(I am ring)+υ(CH2(N))+υ(CH3) (Triangles). (B) Lowest energy geometries calculated for (EMIM)+ at −1.0 and −1.7 V on Cu(100) (top and side views) indicating the preference of the parallel orientation at a more negative potential. Atom color code: blue = N; cyan=C; white = H. Credit: Modified chemistry (2023). DOI: 10.1002/ange.202312163
Case Western Reserve University researchers are developing ways to convert waste into fuels and other products, using energy-efficient processes powered by renewable sources.
Specifically, they are close to solving the challenge of converting carbon dioxide (CO2), a major greenhouse gas, into valuable chemicals using electricity.
CO2 can be a useful raw material for making basic chemicals and fuels. But the process of creating the necessary reaction is not easy because it requires high pressures and temperatures and special materials.
“Our modern society has a crucial need for technologies capable of capturing CO2 waste – or even air – and convert it into products under harmless conditions,” said Burcu Gurkan, professor of chemical engineering at the Case School of Engineering. “The electrochemical conversion of carbon dioxide is an unsolved problem which dates back over 150 years.”
Research to date has mainly focused on developing catalytic materials and understanding energy-intensive CO emissions.2 conversion reaction in water-based electrolytes. Yet challenges remain because water-based systems have limited CO production capacity.2. Additionally, the process includes unwanted side reactions, such as hydrogen gas emissions.
But in a study published this fall in the European journal Modified chemistrythe Case Western Reserve research team demonstrated that the ionic liquids they developed effectively capture and convert CO2 in an electrochemical process.
Ionic liquids are salts that melt below 100°C. Those developed by Gurkan’s group are liquid at room temperature. These ionic liquids are also unique in that they have a high CO production capacity2 capture and maintain electrochemical stability. As a result, the team achieved the desired electrochemical process.
“Our approach focuses on ionic liquid electrolytes that can change thermodynamics and product distribution due to kinetic effects that can be further tuned, thanks to the flexibility of ionic liquid design,” Gurkan said.
The study, led by Oguz Kagan Coskun, a doctoral student in Gurkan’s group, combined spectroscopic and electroanalytical techniques to reveal the fundamental mechanisms needed for ionic liquids to activate CO.2 reduction reaction on the surface of the copper electrode.
The group said it needed less energy to drive the reaction and noted that this could lead to the creation of a variety of industrially relevant products, without the unwanted side products found in the traditional electrolysis process.
Furthermore, the report explains the crucial aspects that influence the properties of the reaction environment for the efficient utilization of CO.2. This additional information contributes to a deeper understanding of the reaction environment, particularly regarding unconventional electrolytes.
The team plans to examine the different reaction steps in more detail to inform subsequent electrolyte designs. The ultimate goal: better control the chemicals resulting from the reaction and advance electrochemical approaches to CO2 recycling.
More information:
Oguz Kagan Coskun et al, Adaptation of electrochemical reduction of CO2 on copper by a reactive ionic liquid and native hydrogen bond donors, Modified chemistry (2023). DOI: 10.1002/ange.202312163
Provided by Case Western Reserve University
Quote: Research aims to convert greenhouse gases into valuable products using electricity (January 4, 2024) retrieved January 5, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.