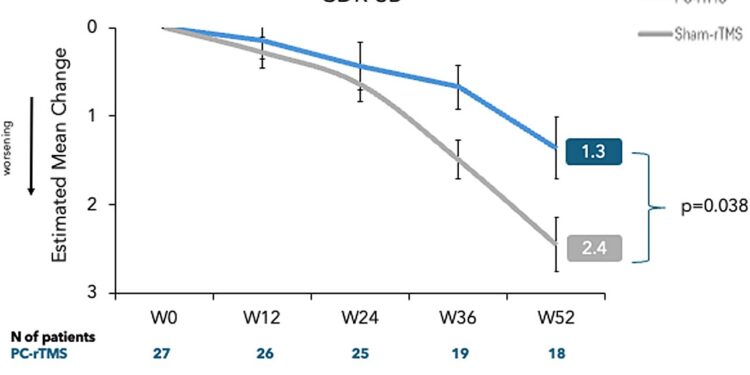
Measurement of primary results. Average group changes estimated for clinical scores. Credit: Alzheimer’s research and therapy (2025). DOI: 10.1186 / S13195-025-01709-7
The research led by the IRCCS of the Santa Lucia Foundation reports that repetitive transcranial magnetic stimulation (RTMS) targeting pre -lap can slow the progression of cognitive decline, daily functioning disorders and behavioral symptoms in patients with Alzheimer’s disease. Patients who received 52 weeks of SMTR have shown a slower deterioration between clinical results compared to those who have received dummy stimulation.
The SMPR provides magnetic impulses to targeted brain areas and is considered a non -invasive brain stimulation technique. It is used to deal with conditions such as depression and obsessive compulsive disorders by inducing small electric currents in brain cells to modify neural activity and help reduce symptoms.
The region of the early brain has been identified as a promising site for stimulation due to its early involvement in Alzheimer’s pathology, including amyloid deposit, loss of gray matter and disturbed connectivity in cerebral networks.
In animal models, the SMTR has been shown to reduce the barte-amyloid and phosphorylated tau, increases neurogenic proteins such as the neurotrophic factor derived from the brain and decreases pro-inflammatory cytokines, especially IL-6 and TNF-α.
A previous phase II test revealed that 24 weeks of SMTR targeted by early slowed down cognitive decline and reduces daily functional capacity loss in patients with light to moderate Alzheimer’s disease. Then, the researchers wanted to see if the extension of the SMTR treatment at 52 weeks could continue to preserve cognition and operation on the longest time.
In the study, “Effects of 52 weeks of pregcuus RTMS in patients with Alzheimer’s disease: a randomized trial”, published in Alzheimer’s research and therapyThe researchers conducted a randomized, randomized and double blind control pilot trial.
The cohort included 48 patients diagnosed with light to moderate Alzheimer’s disease. In total, 27 patients were allocated to receive SMTR and 21 were assigned to a fake stimulation group. Among them, 31 continued from a previous 24 -week test which used the same experimental conception, and 17 were newly registered and randomized.
Continuous participants remained in their original treatment group and extended their participation by 28 additional weeks, which gave its total duration of SMTR or dummy exposure to 52 weeks. The newly registered participants began to be treated at the start of the test and took a full 52 -week course, which included an intensive two -week phase followed by weekly maintenance sessions.
The stimulation has been delivered using a neuronavigated transcranial magnetic stimulation associated with an electroencephalography (MSD-EEG) to personalize the location and the intensity for each participant. Each session involved 40 trains of two seconds at 20 Hz spaced in 20 minutes, totaling 96,000 pulses per patient throughout the test.
Patients who have received repetitive transcranial magnetic stimulation have experienced a slower rate of cognitive and functional decline compared to those of the simulated group. The main result, measured by the change in the clinical dementia assessment scale – Summer of boxes, showed an estimated average change of 1.36 points in the stimulation group against 2.45 points in the simulated group after 52 weeks.
The functional capacity, evaluated using the cooperative study scale of Alzheimer’s disease for the activities of daily life, decreased by around 1.5 points in the RTMS group and 11.6 points in the Sham group.
The cognitive performance, measured by the cognitive sub-cheer of the Alzheimer’s disease assessment scale, showed an average increase of 5.9 points in the RTMS group against 10.4 points in the simulated group. In this context, higher scores reflect greater disability.
The scores on the mental state examination decreased 1.1 points in the RTMS group and 3.9 points in the Sham group. The behavioral symptoms, measured with the neuropsychiatric inventory, also favored the RTMS group, with an estimated change of 3.28 points against 6.91 points in the simulation. The sub-cheaks have shown measurable improvements in apathy, euphoria and disruption linked to appetite.
The completion rate between the two groups was 68%. Most of the withdrawals of the participants were linked to the disturbances caused by the COVVI-19 pandemic. Light undesirable effects such as headaches or discomfort of the scalp have been reported in the two groups and resolved without medical intervention.
Stronger basic connectivity in the brain’s default fashion network was associated with a more favorable clinical response in the RTMS group, suggesting that the profiling of brain activity could help identify the patients most likely to benefit from this treatment.
Researchers have concluded that the SMTR targeting the precuneus can reduce the progression of cognitive decline in patients with light to moderate Alzheimer’s disease. The treatment also seemed to delay the deterioration of daily functioning and reduce behavioral disturbances. Patients receiving SMTRs have not shown a significant loss of autonomy over the period of one year, as measured by standardized assessments.
Certain behavioral symptoms, including apathy, euphoria and changes related to appetite, have also improved in patients receiving RTMS. Less reductions in daily life activities can result in a lower load and greater independence of patients during the early stages of diseases.
More important studies on several sites are necessary to confirm these results and could also explore how SMTR could be used in combination with drug therapies that target the amyloid, tau or neuroinflammation.
More information:
Giacomo Koch et al, effects of 52 weeks of RTMS Pre -En in patients with Alzheimer’s disease: a randomized trial, Alzheimer’s research and therapy (2025). DOI: 10.1186 / S13195-025-01709-7
© 2025 Science X Network
Quote: Repetitive transcranial magnetic stimulation is promising in the treatment of Alzheimer’s (2025, April 15) recovered on April 15, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.