Local administration of exogenous Tregs promotes healing of injured tissues in mice. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-51353-2
Researchers are constantly seeking to identify new therapeutic approaches for regenerative medicine. Recent strategies have focused on harnessing the power of the body’s tissue healing and repair mechanisms, including anti-inflammatory signaling molecules and immune cells.
In collaboration with Professor Shizuo Akira of IFReC, a research team led by Associate Professor Mikaël Martino of Monash University, who also held a cross-appointment at Osaka University, recently published a significant advance in regenerative medicine in Nature Communications.
In the paper, the researchers describe a potential new method involving the administration of regulatory T cells (Tregs) to enhance tissue healing.
After tissue injury, some immune cells trigger a pro-inflammatory response. To prevent chronic inflammation and further damage or disease, a shift to an anti-inflammatory response is needed to complete the healing process.
Although scientists have previously attempted to support regenerative medicine by modulating a patient’s own immune cells, recent developments have seen researchers test the effects of administering specific cell types that can regulate both the immune system and tissue healing.
“We began to explore the use of Tregs for regenerative medicine because they can directly impact other types of immune cells called monocytes and macrophages,” says Mikaël Martino, lead author of the study. “In addition, Tregs can secrete signaling molecules that promote tissue healing. Despite their great potential, few studies have explored the use of Tregs for such applications.”
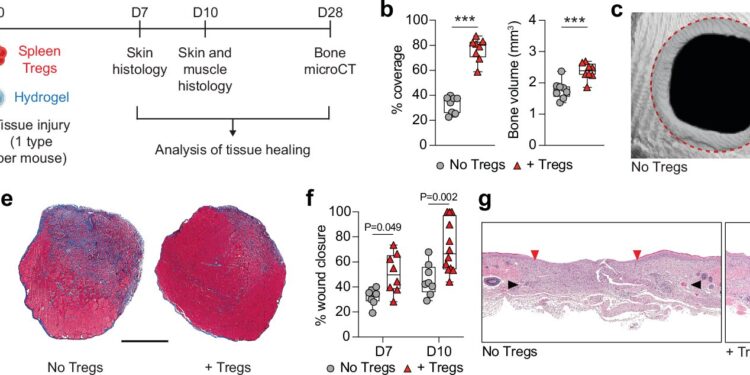
In this study, the team used a mouse model to simultaneously examine bone regeneration, muscle regeneration, and skin repair, ensuring that the injuries were severe enough to require therapeutic intervention. Using a cell delivery method called fibrin hydrogel, they then locally delivered Tregs to the injured areas.
“Compared to those given fibrin hydrogel without Tregs, mice given Tregs showed increased bone volume and coverage over injured skull areas, greater amounts of muscle tissue and larger muscle fiber size, and faster closure of skin wounds,” said Shizuo Akira, lead author of the study.
Further mechanistic investigations showed that administered Tregs adopt a lesion-specific phenotype once in the damaged area. In doing so, Tregs display increased expression levels of genes related to immunomodulation and tissue healing.
Further experiments demonstrated that Tregs can induce monocytes and macrophages in these tissues to switch to an anti-inflammatory state, including by secreting signaling molecules such as interleukin-10 (IL-10).
“Interestingly, we observed that when the gene encoding IL-10 is knocked out of Tregs, their healing effects are lost,” Martino says. “This finding points to the key role of IL-10 in how these Tregs promote tissue repair and regeneration.”
Overall, this study demonstrates the strong potential of using Tregs as a cell therapy for regenerative medicine. These data will help develop innovative treatment methods that can promote tissue healing.
More information:
Bhavana Nayer et al, Local administration of regulatory T cells promotes tissue healing, Nature Communications (2024). DOI: 10.1038/s41467-024-51353-2
Provided by Osaka University
Quote:Regulatory T cells enhance tissue healing in mouse model (September 11, 2024) retrieved September 11, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.