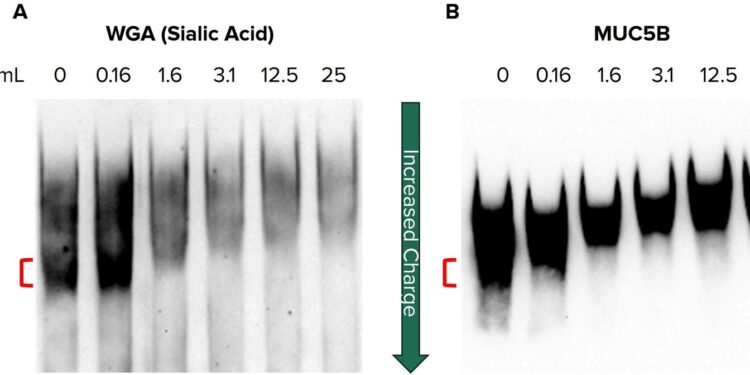
Reduced sialylation of secreted mucin contributes to a low-burden form of MUC5B. Western blots on agarose-PAGE of partially purified mucin from non-CF HBEC secretions. Mucin was treated with increasing concentrations of neuraminidase, ranging from 0 to 25 mu/mL, to remove sialic acid and separated by gel electrophoresis before being probed for (A) sialic acid (WGA) and (B) MUC5B. Credit: Scientific reports (2024). DOI: 10.1038/s41598-024-66510-2
With every breath a human can inhale thousands of harmful microbes into their lungs. Mucus, the moist, gelatinous substance that lines the airways, is one of the first lines of defense and helps clear these microbes. It traps bacteria, viruses, dust and pollen to protect the lungs, and the mucus is moved up and out of the airways by the beating of tiny hair-like projections called cilia.
But what happens when the body produces too much mucus—it’s too thick, too sticky, and too dehydrated to move and clear properly? Over time, mucus that’s too thick and sticky can clog the airways, disrupting mucus clearance mechanisms and creating a breeding ground for trapped microbes. These pathophysiological processes contribute to the development of mucoobstructive diseases, including cystic fibrosis, or CF.
A University of Alabama at Birmingham research team led by Jarrod W. Barnes, Ph.D., associate professor in the UAB Department of Medicine, and Steven M. Rowe, MD, professor in the Division of Pulmonary, Allergy and Critical Care Medicine in the UAB Department of Medicine, studied the defective mucus formation. They show in research published in Scientific reports how a single change in sugar on mucus affects its expansion and transport.
Mucus is a hydrogel composed primarily of water and solids, including large sugar-coated protein molecules called mucins, the main component of mucus. Research has focused on adding and removing a negatively charged sugar terminal modification, called sialic acid, from MUC5B, one of the major mucin proteins found in the airways. A high anion density of MUC5B promotes mucus polymerization and packaging, but previous reports have shown a less negatively charged “low-charged” form of mucin in cystic fibrosis and asthma.
UAB researchers found that reducing sialic acid levels of MUC5B was sufficient to generate this low-charged, potentially pathological form.
When the researchers reduced the sialic acid on MUC5B, they observed a change in the protein’s electrophoretic mobility, indicating a less charged form. Transmission electron microscopy imaging showed that the reduced sialylation contributed to the tangling (more compact) of MUC5B polymers compared to normal levels of mucin sialylation that facilitate linearization and expansion.
Reducing negative charge, by specifically blocking α-2,3-sialylation, influenced mucus structure and affected its transport in human bronchial epithelial cells and in rat trachea models of intact airways. Thus, the authors report that “mucin load may have pathological significance in mucoobstructive diseases through increased mucus compaction and impaired transport.”
The study also found that CF patients had reduced expression of an enzyme called ST3Gal1, which adds α-2,3 sialic acid to mucin proteins. However, treatment with the cystic fibrosis transmembrane conductance regulator protein correctors elexacaftor, tezacaftor, and ivacaftor partially restored ST3Gal1 expression.
By showing a crucial role of mucin sialylation on mucus properties and transport, the study identified a possible therapeutic strategy for the treatment of cystic fibrosis and potentially other debilitating mucoobstructive diseases, such as chronic obstructive pulmonary disease, or COPD, primary ciliary dyskinesia and non-cystic fibrosis bronchiectasis.
More information:
Elex S. Harris et al., Reduced sialylation of airway mucin impairs mucus transport by altering mucin biophysical properties, Scientific reports (2024). DOI: 10.1038/s41598-024-66510-2
Provided by the University of Alabama at Birmingham
Quote:Reduced sialylation of mucin impairs mucus transport in the lungs, study finds (2024, September 24) retrieved September 24, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.