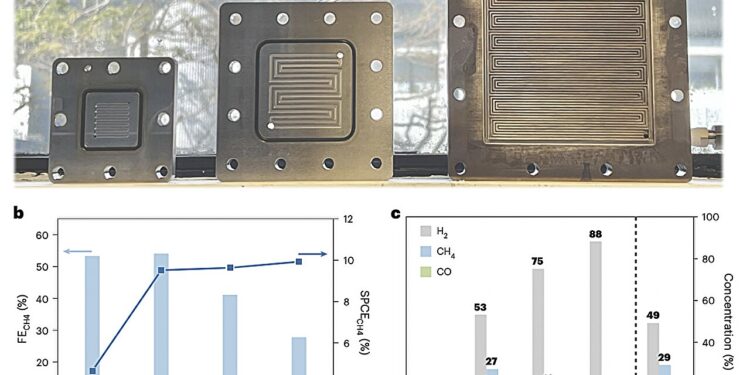
Large-scale molecular electrode to supply energy. a, Photograph of the MEA electrolyzer at 4, 20 and 81 cm2, from left to right. b, FE and carbon efficiency in a single pass from CO2 to CH4 on an 81 cm2 DAT electrode under a total current of 10 A (current density = 123 mA cm−2) with different CO2 flow rates. c, Concentration of gaseous products on an 81 cm2 DAT electrode under a total current of 10 A (current density = 123 mA cm−2) with different CO2 flow rates. Credit: Xu et al. (Nature Energy, 2024).
The efficient conversion of carbon dioxide (CO2) – one of the main compounds contributing to climate change – into useful fuels and chemicals is a long-sought research goal. Recent studies have introduced various catalysts that could be used to initiate so-called electrochemical CO.2 reduction reaction in electrolyzers (i.e. devices that drive specific chemical reactions using electricity).
The CO2 The reduction reaction is the chemical process by which CO2 the molecules are reduced, eventually forming other chemicals or fuels. Most catalysts previously used to initiate this reaction in electrolyzers are metallic, such as copper, silver and gold.
However, metal-based catalysts often have limited adaptability, meaning there is a need to harness them to precisely reduce CO2 in specific chemicals can be difficult. Some studies have thus evaluated the potential of alternative non-metallic catalysts to convert CO2 into desirable fuels and chemicals.
Researchers from the Chinese University of Hong Kong, the University of Auckland and National Yang Ming Chiao Tung University recently presented a promising new triazole molecular catalyst for the efficient electrochemical reduction of CO2 in methane (CH4). A preliminary system using this catalyst, presented in an article published in Natural energyproven to reliably convert CO2 in CH4presenting both good efficiency and a good rotation frequency.
“Organic molecular catalysts, which are more precise than metal catalysts, are still unable to catalyze CO2 to hydrocarbons under industrially relevant current densities for long-term operation, and the catalytic mechanism is still elusive,” Zhanyou Xu, Ruihu Lu and colleagues wrote in their paper. “We report 3,5-diamino-1 ,2,4- triazole membrane electrode assemblies for CO2-to-CH4 conversion with a faradic efficiency of (52 ± 4)% and a rotation frequency of 23,060 h−1 at 250 mA cm−2“.
Researchers have imagined a first CO reduction system2 using their triazole molecule catalyst and explored its performance in a series of tests, where they ran it at a current of 10 A for 10 hours of electrolysis. Their findings were very promising and gave them valuable insight into the process by which their system converts CO2 in CH4.
“Our mechanistic studies suggest that CO2 the reduction at the 3,5-diamino-1,2,4-triazole electrode takes place via *CO2–*COOH–*C(OH)2–*COH to produce CH4 due to the spatially distributed active sites and the appropriate energy level of the molecular orbitals,” Xu, Lu and colleagues wrote. “A pilot system operated under a total current of 10 A (current density = 123 mA cm−2) for 10 h is capable of producing CH4 at a rate of 23.0 mmol h−1“.
Overall, the results of this recent study highlight the potential of triazole molecular catalysts to enable scalable and selective CO electroreduction.2. The promising catalyst they identified, 3,5-diamino-1,2,4-triazole (DAT), could soon be further studied and evaluated by other research teams, or could inspire the design of similar catalysts to convert CO2 into other useful chemicals.
More information:
Zhanyou Xu et al, Electroreduction of CO2 methane with triazole molecular catalysts, Natural energy (2024). DOI: 10.1038/s41560-024-01645-0.
© 2024 Science X Network
Quote: Promising triazole molecular catalyst enables efficient electroreduction of carbon dioxide to methane (October 22, 2024) retrieved October 22, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.