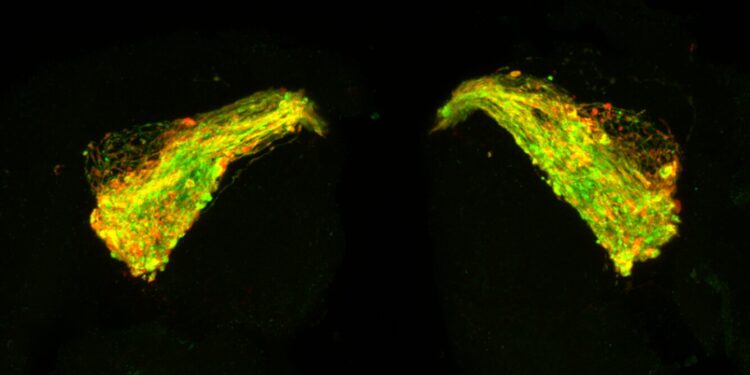
The brain of a 7-day-old fruit fly with Tau expressed in a neural circuit used by the fly in olfactory memory. Green describes neurons that begin to swell and degenerate because of the Tau protein. Red shows where Tau accumulates in clusters along neurons, starting to form clumps that eventually become string-like fibrils. Credit: University of Southampton
An international team of researchers led by Lancaster University has made a promising breakthrough in the development of drugs to treat Alzheimer’s disease. For the first time, scientists have developed a drug that acts on the two main “hot spots” that promote the aggregation of tau protein in the brain, a key factor in neurodegeneration.
The drug, a peptide inhibitor called RI-AG03, has been shown to prevent the accumulation of tau proteins in laboratory and fruit fly studies.
The research, published in Alzheimer’s and dementiawas led by Lancaster University in collaboration with the University of Southampton, Nottingham Trent University, Tokyo Metropolitan Institute of Medical Sciences and the University of Texas South West Medical Centre.
The Lancaster University team included the late Professor David Allsop and the late Dr Nigel Fullwood, both from the Faculty of Biomedical and Life Sciences at Lancaster University.
The article describes how RI-AG03 was first developed by Dr Anthony Aggidis in the laboratory of the late Professor Allsop, using computational biology, where it was tested in laboratory dishes.
Lead author Dr Aggidis, a former postdoctoral research associate at Lancaster University and visiting researcher at the University of Southampton, said: “Our research represents an important step towards creating treatments that can prevent progression diseases such as Alzheimer’s disease. key areas of tau protein, this unique approach could help address the growing impact of dementia on society, providing a much-needed new option for treating these devastating diseases.
A significant advance
Tau proteins play a crucial role in maintaining the structure and function of neurons (brain cells). But in Alzheimer’s disease, these proteins malfunction and clump together to form long, twisted fibrils. As the fibrils build up, they create what are called neurofibrillary tangles: masses of twisted tau proteins that obstruct neurons, preventing them from getting the nutrients and signals they need to survive.
As more and more neurons die, memory, thinking, and behavior become increasingly impaired, leading to the cognitive decline seen in Alzheimer’s disease.
There are two specific tau protein “hot spots” where this clumping tends to occur. While current treatments target either of these hot spots, RI-AG03 uniquely targets and blocks both.
Amritpal Mudher, professor of neuroscience at the University of Southampton, said: “There are two regions of the tau protein that act like a zipper to allow it to aggregate. For the first time, we have an effective drug to inhibit these two proteins. This dual targeting mechanism is important because it addresses both areas that drive Tau aggregation, potentially paving the way for more effective treatments for neurodegenerative diseases like Alzheimer’s.
The scientists used a special imaging technique to observe how the Tau protein clumps together in the brain tissue of genetically modified flies in response to treatment with RI-AG03. Without the treatment (A), they saw toxic clumps of Tau fibrils (arrow) and oligomers (arrowhead). After treatment (B), these harmful clumps have disappeared and only large round structures remain, which scientists believe are less harmful. Credit: University of Southampton
Targeted approach
The peptide-based approach is also more targeted than current treatments, potentially making it safer and causing fewer side effects.
Dr Aggidis said: “We know that the toxicity of tau is closely linked to its ability to aggregate. Thus, by inhibiting aggregation, we expect to see desirable effects. But current aggregation inhibitors have had many side effects because they can interfere with aggregation. functions of many other proteins. RI-AG03 is specifically designed against tau, meaning it is less likely to interact unwantedly with other proteins.
RI-AG03 review
To test its effectiveness in the cells of a living organism, researchers at the University of Southampton then administered the drug to fruit flies with pathogenic tau protein. These fruit fly models of Alzheimer’s disease were generated by Dr Shreyasi Chatterjee, a senior lecturer at Nottingham Trent University.
The researchers found that the drug suppressed neurodegeneration and extended the flies’ lives by about two weeks, a significant extension considering the lifespan of the insects.
To understand what was happening, Southampton scientists studied the brains of fruit flies in depth.
Professor Mudher said: “When we didn’t feed the flies the peptide inhibitor, they had lots of pathogenic fibrils, which cluster together to form a tangle. But when we fed them the drug, the pathogenic fibrils decreased significantly in the higher the dose given, the more improvement we saw in the lifespan of the fruit fly.
To make sure it wasn’t unique to fruit flies, researchers at the University of Texas Southwestern Medical Center tested the drug in a biosensor cell, a type of living human cell line designed to detect the formation of pathogenic tau fibrils. Here too, they found that the drug successfully entered cells and reduced tau protein aggregation.
The team believes their work will have a significant impact on drug discovery efforts in the field of neurodegenerative diseases and now plans to test RI-AG03 in rodents, before proceeding to clinical trials.
Dr Richard Oakley, associate director of research and innovation at the Society, said: “Dementia is the biggest cause of death in the UK, and it places huge costs and pressures on our healthcare system. health… This research takes promising steps towards a new health system. first-of-its-kind therapy that targets tau, a damaging protein in the brains of people with Alzheimer’s disease, preventing it from clumping together. This drug has the potential to be more targeted than others currently being studied, and we hope this will result. with fewer toxic side effects.
“It’s important to note that the study is in its early stages, so we don’t yet know if it will work or be safe for humans, but it’s an exciting development and we look forward to it. to see where this will take us.
“Research will beat dementia, but we need to make it a reality sooner with more funding, more partnerships and more people participating in dementia research.”
More information:
A novel peptide-based inhibitor of tau aggregation as a potential treatment for Alzheimer’s disease and other tauopathies, Alzheimer’s and dementia (2024). DOI: 10.1002/alz.14246
Provided by Lancaster University
Quote: Promising Alzheimer’s drug candidate prevents tau protein buildup in lab and fruit fly studies (October 3, 2024) retrieved October 3, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.