Credit: Joule (2023). DOI: 10.1016/j.joule.2023.08.009
Polymer-air batteries often face challenges related to stability, kinetics and conductivity. In response, Dr. Jodie Lutkenhaus developed a method to use a polymer as an anode in these batteries.
In a recent article published in JouleLutkenhaus, associate head of the internal engagement department and professor of chemical engineering at Texas A&M University, collaborated with chemical engineering professor Dr. Abdoulaye Djire to reveal how these polymers store and exchange charges with the electrolyte.
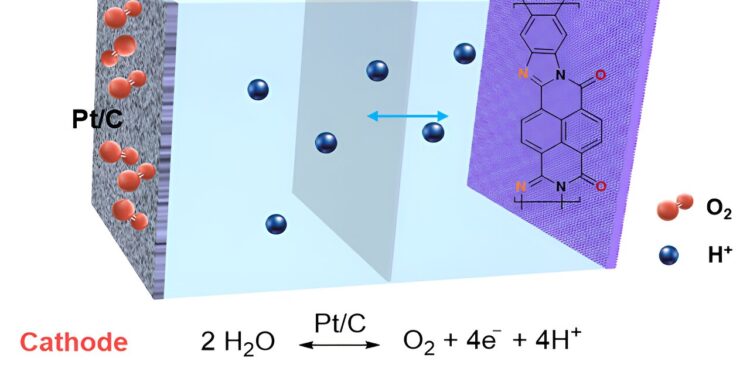
“The cathode reacts with oxygen in the air to complete the circuit. We specifically targeted the use of a conjugated polymer with a rigid base structure for the anode,” Lutkenhaus said.
These characteristics make the polymer both conductive and stable, enabling the reversible reactions needed for repeated charging and discharging, she said.
Despite the advantages of aqueous polymer-air batteries, including improved safety, reduced cost, higher ionic conductivity and increased durability, their electrochemical performance is limited, the article states.
“To overcome these limitations, researchers have explored alternative polymer anodes, which have several advantages over metal anodes, including low cost, ease of functionalization, and high stability,” the researchers write.
The battery’s rigid ladder structure, fast kinetics and high electrical conductivity allowed it to endure 500 cycles with minimal performance loss. Furthermore, the paper reveals the real-time charge transfer mechanism, demonstrating a rapid charge compensation process of hydronium ions.
The article notes that metal-air batteries have a higher energy density than conventional lithium-ion batteries. This is attributed to the oxygen cathode, which offers much higher capacity than conventional metal oxide cathodes. However, the article highlights the limitations of using metal anodes in air batteries due to the stability, cost and environmental impact of mining and processing metal resources.
Issues such as dendrites, passivation, and corrosion on the metal anode lead to low utilization and lower cycling stability in metal-air batteries. Despite the adoption of interface and electrolyte formulation modifications, these problems can be severe in the presence of air oxygen.
“The polymer-air battery offers an alternative means of energy storage compared to the metal-air battery,” Lutkenhaus said. “The polymer-air battery has a large energy storage capacity and a very long service life.”
She explained that having a long lifespan means people can use their batteries longer before needing to recharge or replace them.
“Air batteries are promising for high-energy batteries because they have lower mass than conventional batteries,” Lutkenhaus said. “However, long-term operation of air batteries often results in the generation of carbonate deposits that clog the battery and prevent long-term operation. By using a polymer as an electrode, we could modify the electrolyte to prevent the formation of carbonates.”
More information:
Ting Ma et al, Understanding the mechanism of a conjugated ladder polymer as a stable anode for polymer-air acid batteries, Joule (2023). DOI: 10.1016/j.joule.2023.08.009
Joule
Provided by Texas A&M University College of Engineering
Quote: Polymer-air battery research studies advanced energy storage solutions (December 21, 2023) retrieved December 22, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.