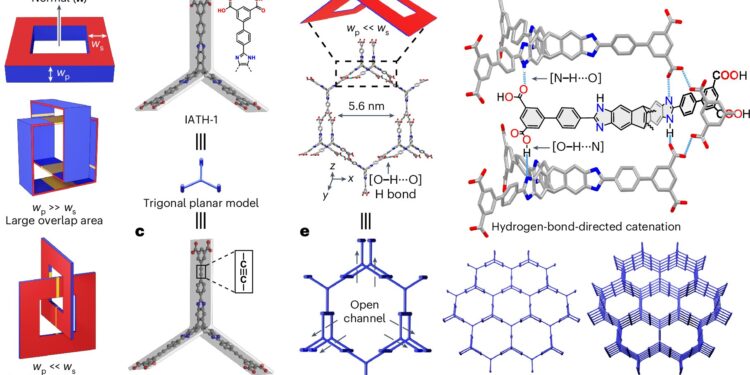
Catenation analysis and crystal superstructures of RP-H100 and RP-H101. Credit: Natural chemistry (2024). DOI: 10.1038/s41557-024-01622-w
Hydrogen is often considered the fuel of the future due to its zero and high gravimetric energy density, meaning it stores more energy per unit mass than gasoline. Its low volumetric density, however, means that it takes up a large amount of space, posing problems for efficient storage and transportation.
To overcome these deficiencies, hydrogen must be compressed in tanks at an extremely high pressure of 700 bars. This situation not only leads to high costs, but also raises security concerns.
To make hydrogen-powered fuel cell vehicles (FCVs) more widespread, the U.S. Department of Energy (DOE) has set specific targets for hydrogen storage systems: 6.5% of vehicle weight storage material must be hydrogen (gravimetric storage capacity of 6.5% by weight). ), and one liter of storage material must contain 50 grams of hydrogen (a volumetric storage capacity of 50 g L‒1). These targets ensure that vehicles can travel reasonable distances without excess fuel.
A promising strategy to achieve these goals is to develop porous adsorbent materials, such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and porous organic polymers (POPs). All of these materials share a common characteristic: they have a porous structure that allows them to effectively trap and store hydrogen gas. This approach also aims to facilitate the storage of hydrogen at a lower pressure, for example less than 100 bars.
Despite progress in exceeding the DOE’s gravimetric goal, many adsorbent materials still struggle to meet volumetric capacity requirements, and few are able to balance volumetric and gravimetric goals. From an industrial perspective, volumetric capacity is more crucial than gravimetric capacity because vehicle storage tanks have limited space.
The volume of a hydrogen storage system directly impacts the autonomy of FCVs. Therefore, it is essential to develop hydrogen adsorbents that maximize volumetric capacity while maintaining excellent gravimetric capacity. Achieving this goal involves balancing high volumetric and gravimetric surface area within the same material.
Researchers are investigating various materials for hydrogen storage, with the assembly of organic supramolecular crystals from organic molecules through non-covalent interactions being a promising option due to their recyclability. Their potential, however, remains largely untapped, because it is difficult to design supramolecular crystals with high and balanced gravimetric and volumetric surfaces, while maintaining their stability.
A phenomenon known as catenation, which involves mechanically interlocking networks in porous materials, generally improves stability. However, catenation often reduces surface area by blocking accessible surfaces, making the material less porous and generally undesirable for hydrogen storage. Efforts are usually made to minimize or avoid it.
Interpenetration analysis of RP-H100 and RP-H101. Credit: Natural chemistry (2024). DOI: 10.1038/s41557-024-01622-w
To unlock the potential of supramolecular crystals for hydrogen storage, a collaborative research team led by Professor Fraser STODDART, with Research Assistant Professors Dr Chun Tang and Dr Ruihua Zhang from the Department of Chemistry at the University of Hong Kong (HKU), and Professor Randall Snurr from the Department of Chemical and Biological Engineering at Northwestern University, USA, demonstrated a controlled “contact point catenation strategy”.
The research is published in the journal Natural chemistry.
This innovative approach uses hydrogen bonds, whose cross-section can be thought of as a “point”, rather than traditional stacking (π···π) which involves a large “area” overlap, to guide catenation in a manner precise in supramolecular crystals. Based on this strategy, the researchers create a well-organized framework that minimizes surface area loss caused by interpenetration and tailors the pore diameter (~1.2 to 1.9 nm) for optimal hydrogen storage. .
As a result, the research team obtained a supramolecular crystal with record gravimetry (3,526 m2 g‒1) and volumetrically balanced (1,855 m2 cm‒3) surfaces among all reported (supra)molecular crystals, in addition to high stability, while (i) providing excellent volumetric capacity at the material level (53.7 g·L‒1), (ii) balancing a high gravimetric capacity (9.3% by weight) for hydrogen storage under practical pressure and temperature variation conditions (77 K/100 bar → 160 K/5 bar), and (iii) exceeding DOE’s ultimate system-level goals. (50 g·L‒1 and 6.5% by weight both volumetric and gravimetric, albeit at cryogenic temperatures.
Innovative design
Designing organic supramolecular crystals that balance high gravimetric and volumetric surface areas, while maintaining high stability, poses a significant challenge, which has hampered its potential for many applications.
The team, however, proposed a point-contact catenation strategy that uses point-contact interactions involving hydrogen bonds to minimize surface area loss during catenation. This design strategy gives these supramolecular crystals high volumetric and gravimetric surface areas, high stability, and ideal pore sizes for hydrogen storage.
This research reveals the potential of organic supramolecular crystals as promising candidates for on-board hydrogen storage and highlights the potential of a directional catenation strategy in designing robust porous materials for applications.
More information:
Ruihua Zhang et al, Balancing the volumetric and gravimetric capacity of hydrogen in supramolecular crystals, Natural chemistry (2024). DOI: 10.1038/s41557-024-01622-w
Provided by the University of Hong Kong
Quote: Organic supramolecular crystals with high hydrogen storage performance could improve the efficiency of fuel cell vehicles (September 27, 2024) retrieved September 27, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.