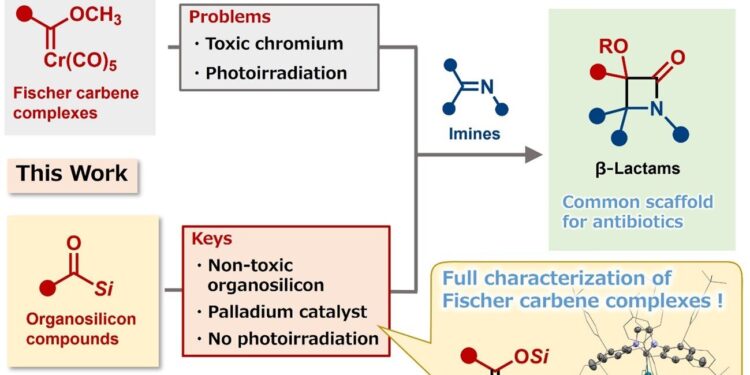
Palladium-catalyzed generation of Fischer carbene complexes and its use for the synthesis of β-lactams. Credit: Mamoru Tobisu
Although we seem to live in a world of abundance, behind the scenes, sourcing sufficient quantities of key ingredients can be difficult. For example, the synthesis of some antibiotics, beta-lactam antibiotics, requires certain molecules in large quantities, and it is historically difficult to obtain enough of these molecules.
In a study titled “Catalytic synthesis of β-lactam derivatives by carbonylative cycloaddition of acylsilanes with imines via a Fischer-palladium carbene intermediate” and published in Natural catalysisOsaka University researchers unveil a new, simplified way to synthesize the complex beta-lactam scaffold characteristic of beta-lactam antibiotics.
Penicillin, the first mass-produced antibiotic, is an example of a beta-lactam antibiotic. These antibiotics are the first choice for some bacterial infections because they are more selective and less toxic than most medications. Not surprisingly, there are dozens of beta-lactam antibiotics approved for clinical use and under development.
Unfortunately, synthesizing these antibiotics in the laboratory has proven difficult due to their small but complex structure. Thus, catalysts are generally required to facilitate critical steps in the synthesis. A catalyst, known as the Fischer-carbene complex, works well but must be used in large quantities. The synthesis of a Fischer-carbene catalyst acting in small quantities was the goal of the research team’s study.
“Our new catalytic system can generate Fischer-carbene complexes from organosilicon compounds, which are non-toxic,” explains Tetsuya Inagaki, lead author of the study. “In addition, we were able to isolate and characterize a key intermediate: a siloxycarbene-palladium complex.”
Unlike previous Fischer-carbene synthesis protocols, the process does not generate toxic chromium waste and does not require photo-irradiation. The reaction occurs in a single step, is operationally simple, and requires only a small amount of catalyst. The researchers used it to prepare the scaffold for the antibiotic thienamycin in 94% yield.
“We are excited because our research will help synthesize Fischer-carbene catalysts that would otherwise be difficult to isolate and provide access to structurally complex beta-lactam building blocks in a single reaction vessel,” said lead author Mamoru Tobisu. “We look forward to applying our reaction protocol to other classes of synthetic targets.”
This work represents an important step in simplifying the synthesis of beta-lactams, the most common molecular structure of antibiotics. The synthesis protocol being simple and low toxicity, applications to other chemical transformations should be simple.
More information:
Tetsuya Inagaki et al, Catalytic synthesis of β-lactam derivatives by carbonylative cycloaddition of acylsilanes with imines via a Fischer-palladium carbene intermediate, Natural catalysis (2024). DOI: 10.1038/s41929-023-01081-5
Provided by Osaka University
Quote: One-step synthesis of the most common, but very complex, antibiotic molecular scaffold (January 16, 2024) retrieved January 16, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.