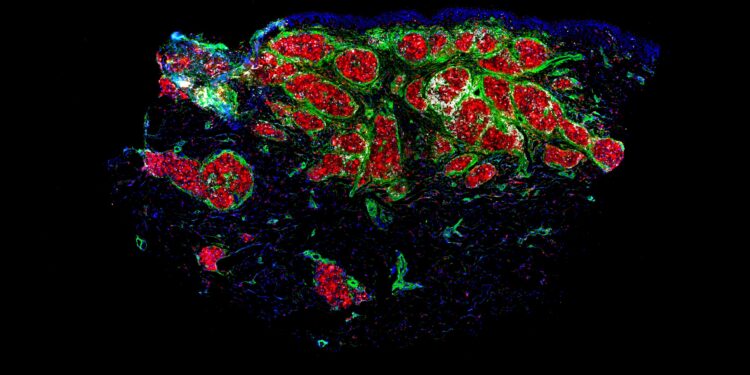
Immunofluorescence staining makes granulomas glow red with a green border (macrophages with fibroblasts). Credit: MedUni Vienna/Anna Redl
A research team led by Georg Stary (Medical University of Vienna and CeMM) has identified a new approach to treating the inflammatory disease sarcoidosis. In a clinical study, inhibition of a specific signaling pathway showed clear success in the treatment of skin granulomas. This opens new therapeutic avenues for sarcoidosis and similar inflammatory diseases.
The results are published in the journal The Lancet Rheumatology.
Sarcoidosis is a difficult-to-treat inflammatory disease that affects about 15 to 20 people in 100,000. In this disease, tiny clumps of inflammatory cells called granulomas form in affected organs. These granulomas can affect normal tissues and lead to inflammation and fibrosis, which ultimately limits the functionality of the affected organs.
In their study, researchers from MedUni Vienna and CeMM (Research Center for Molecular Medicine of the Austrian Academy of Sciences) focused their attention on possible new therapeutic approaches for sarcoidosis.
They chose the mTOR (mechanistic target of rapamycin) signaling pathway, known to regulate the metabolism and growth of many cell types, because mTOR activation was observed in granulomas of different tissues from several patients. Sixteen patients with granulomas of the skin and other organs were included in a clinical study at the Department of Dermatology.
Researchers used the mTOR inhibitor sirolimus, which was first approved in 1999 to prevent organ rejection after kidney transplantation. It had already shown promise in preclinical models of sarcoidosis.
Complete remission of symptoms in some cases
Sirolimus was first administered topically (as a cream) and then systemically (as an oral solution) to test its effect on skin granulomas. Topical treatment has been ineffective, perhaps because granulomas (compact structures in the skin) are difficult to penetrate from the surface.
In contrast, systemic treatment was successful in 7 out of 10 patients who completed the study, and some even experienced complete regression after four months of treatment and no recurrence up to two years later.
Interestingly, patients who responded to systemic therapy had higher mTOR expression in their granuloma fibroblasts than those who did not respond.
“We suspect that, unlike broad-spectrum immunosuppressants, mTOR inhibition targets both immune and non-immune cells in granulomas, which prevents recurrence of tissue granulomas,” explains study leader Georg Stary. .
Multicenter study to confirm results
The clinical study also suggests an effect of systemic treatment on granulomas of other vital organs, although it is difficult to draw concrete conclusions due to the small number of patients.
Researchers are now planning a multicenter clinical trial with more patients to confirm the skin findings and further test the drug’s effectiveness, particularly in lung involvement, present in 90% of people with sarcoidosis.
“Given the rarity of sarcoidosis and the fact that mTOR inhibitors such as sirolimus are no longer patented, industry interest in research is limited. This shows the importance of investigator-initiated studies and of academic research”, says Georg Stary, emphasizing the academic nature of this study.
In future work, the researchers want to study the importance of mTOR and other signaling pathways in other non-infectious granulomatous skin diseases such as necrobiosis lipoidis, often associated with diabetes. Stary hopes their research will lead to new targeted therapies for these neglected diseases.
More information:
Anna Redl et al, Efficacy and safety of mTOR inhibition in cutaneous sarcoidosis: a single-center trial, The Lancet Rheumatology (2024). DOI: 10.1016/S2665-9913(23)00302-8
Provided by the Medical University of Vienna
Quote: New therapeutic approach for the treatment of sarcoidosis (January 23, 2024) retrieved on January 23, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.