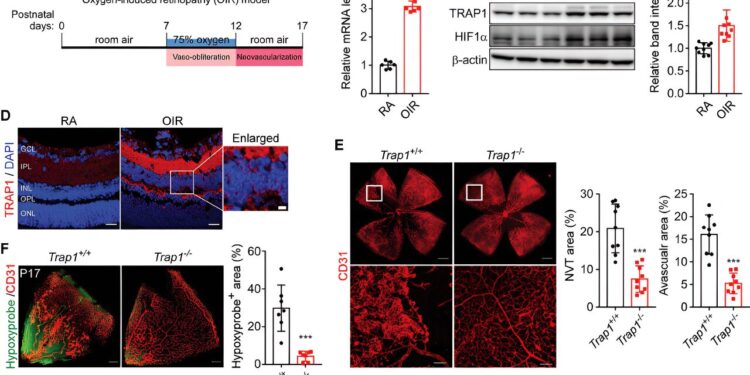
Contribution of TRAP1 to the development of ischemic retinopathy. (A) Schematic of experimental procedures of OIR mice. B) Quantification of TRAP1 mRNA. C) Quantification of TRAP1 protein levels. D) Immunohistochemical staining of TRAP1 in mouse retinas. E) Normalized retinal blood vessels in Trap1−/− OIR mouse. Credit: Advanced science (2023). DOI: 10.1002/advs.202302776
Technology with the potential to treat ischemic retinopathy in premature babies and diabetic patients has been developed by Professor Byoung Heon Kang and his research team from the Department of Biological Sciences at UNIST, in collaboration with Professor Dong Ho Park’s team from Kyungpook National University Hospital.
Ischemic retinopathy, characterized by breakdown of the blood-retinal barrier and abnormal growth of blood vessels, often leads to vision impairment and loss. Researchers have identified the critical role of a mitochondrial chaperone called tumor necrosis factor receptor-associated protein 1 (TRAP1) in the pathogenesis of ischemic retinopathy.
Through genetic ablation of Trap1 or treatment with small molecule TRAP1 inhibitors, such as mitoquinone (MitoQ) and SB-U015, the research team successfully attenuated retinal pathologies in mouse models mimicking ischemic retinopathy.
This therapeutic effect was attributed to the proteolytic degradation of hypoxia-inducible factor 1α (HIF1α), a transcription factor involved in blood-retinal barrier breakdown and pathological neovascularization. Degradation of HIF1α was facilitated by opening of the mitochondrial permeability transition pore and activation of the calcium-dependent protease calpain-1.
The results of this research are published in Advanced science.
These findings open new possibilities for innovative treatments for ischemic retinopathy, including retinopathy of prematurity and proliferative diabetic retinopathy. The technology focuses on targeting and regulating aberrant HIF1α and mitochondrial activation under hypoxic conditions, providing a transformative approach to addressing the underlying causes of retinal diseases. Unlike conventional treatment methods, this technology can be easily administered using ophthalmic medications, making it accessible to a wider range of patients.
Inhibition of TRAP1 triggers calcium/calpain-1-dependent degradation of HIF1α. Above is the schematic image showing HIF1α degradation following TRAP1 inhibition. Credit: Advanced science (2023). DOI: 10.1002/advs.202302776
“Excessive production of angiogenic factors in retinopathy is closely related to mitochondrial properties,” explains Professor Kang. “By suppressing the expression of TRAP1 protein, we can improve the condition of retinopathy.”
The therapeutic substance, currently being developed by Smartin Bio Inc., a startup founded by Professor Byoung Heon Kang, is undergoing non-clinical trials.
The successful development of this technology holds great promise in revolutionizing the therapeutic landscape of ischemic retinopathy, providing both exceptional efficacy and usability that exceeds the limitations of existing treatments. As clinical trials and development progress, this innovation brings hope for a better future for patients suffering from retinopathy.
More information:
So‐Yeon Kim et al, Targeting the mitochondrial chaperone TRAP1 attenuates vascular pathologies in ischemic retinopathy, Advanced science (2023). DOI: 10.1002/advs.202302776
Provided by Ulsan National Institute of Science and Technology
Quote: New technology offers promising treatment for ischemic retinopathy (January 17, 2024) retrieved January 17, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.