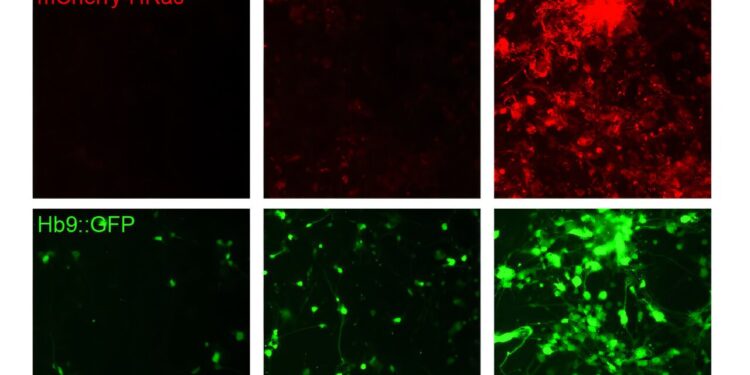
MIT engineers have developed a way to set gene expression levels to off, low, or high. Using skin cells, the researchers created a cocktail (labeled with a red fluorescent protein, top row) that stimulates the conversion of skin cells into motor neurons. Via Promoter Editing, they show that higher levels of this cocktail increase the number of motor neurons (green). In the bottom row, the same cells are labeled with a green fluorescent protein generated after the cells convert into motor neurons. Credit: Natural biotechnology (2025). DOI: 10.1038/s41587-025-02854-y
For decades, synthetic biologists have been developing genetic circuits that can be transferred into cells for applications such as reprogramming a stem cell into a neuron or generating a protein that could help treat a disease such as fragile X syndrome.
These genetic circuits are usually introduced into cells by carriers such as non-pathogenic viruses. However, it has been difficult to ensure that these cells end up producing the correct amount of protein encoded by the synthetic gene.
To overcome this obstacle, MIT engineers designed a new control mechanism that allows them to establish a desired protein level, or set point, for any genetic circuit. This approach also allows them to change the set point after the circuit is delivered.
“This is a really stable, multifunctional tool. The tool is very modular, so you can control many transgenes with this system,” says Katie Galloway, assistant professor of chemical engineering at MIT and lead author of the new study.
Using this strategy, the researchers showed that they could induce cells to generate constant levels of target proteins. In one application they demonstrated, they converted mouse embryonic fibroblasts into motor neurons by delivering high levels of a gene that promotes this conversion.
MIT graduate student Sneha Kabaria is the lead author of the paper, published today in Natural biotechnology. Other authors include Yunbeen Bae ’24; MIT graduate students Mary Ehmann, Brittany Lende-Dorn, Emma Peterman and Kasey Love; Adam Beitz Ph.D. ’25; and former MIT postdoc Deon Ploessl.
Increase gene expression
Synthetic genetic circuits are designed to include not only the gene of interest, but also a promoter region. At this site, transcription factors and other regulators can bind, thereby activating the expression of the synthetic gene.
However, it is not always possible to get all cells in a population to express the desired gene at a uniform level. One reason for this is that some cells may occupy only one copy of the circuit, while others receive many more. Additionally, cells have natural variations in the amount of proteins they produce.
This has made reprogramming cells difficult because it is difficult to guarantee that each cell in a population of skin cells, for example, will produce enough of the transcription factors needed to successfully transition to a new cellular identity, such as a neuron or an induced pluripotent stem cell.
In the new paper, the researchers designed a way to control gene expression levels by changing the distance between the synthetic gene and its promoter. They found that when there was a longer DNA “spacer” between the promoter region and the gene, the gene would be expressed at a lower level. This extra distance, they showed, makes it less likely that promoter-bound transcription factors effectively activate gene transcription.
Then, to create set points that could be changed, the researchers incorporated sites into the spacer that can be excised by an enzyme called Cre recombinase. When parts of the spacer are cut out, it helps bring transcription factors closer to the gene of interest, resulting in gene expression.
The researchers showed that they could create spacers with multiple excision points, each targeted by different recombinases. This allowed them to create a system called DIAL, which they could use to establish “high,” “medium,” “low,” and “off” set points for gene expression.
Once the DNA segment carrying the gene and its promoter is delivered into the cells, recombinases can be added to the cells, allowing the set point to be changed at any time.
The researchers demonstrated their system in mouse and human cells by gene delivery of different fluorescent proteins and functional genes, and showed that they could achieve uniform expression in a population of cells at the target level.
“We achieved uniform and stable control. This is very exciting for us because the lack of uniform and stable control is one of the things that limits our ability to build reliable systems in synthetic biology. When there are too many variables that affect your system, and then you add normal biological variation, it is very difficult to build stable systems,” says Galloway.
Cell reprogramming
To demonstrate the potential applications of the DIAL system, the researchers then used it to deliver different levels of the HRas gene.G12V to mouse embryonic fibroblasts. This HRas variant has previously been shown to increase the rate of conversion of fibroblasts into neurons. The MIT team found that in cells that received a higher dose of the gene, a greater percentage of them were able to successfully turn into neurons.
Using this system, researchers now hope to carry out more systematic studies on different transcription factors that can induce the transition of cells to different cell types. Such studies could reveal how different levels of these factors affect the success rate and whether changing the levels of transcription factors could change the type of cell generated.
In ongoing work, the researchers have shown that DIAL can be combined with a system they previously developed, known as ComMAND, which uses a feedback loop to prevent cells from overexpressing a therapeutic gene.
Using these systems together, it might be possible to tailor gene therapies to produce specific and consistent protein levels in each patient’s target cells, the researchers say.
“This is something we’re excited about because DIAL and ComMAND are highly modular, so you could not only have a well-controlled gene therapy that is somewhat general for a population, but you could, in theory, tailor it to any given person or any given cell type,” Galloway says.
More information:
Sneha R. Kabaria et al, Programmable promoter editing for precise control of transgene expression, Natural biotechnology (2025). DOI: 10.1038/s41587-025-02854-y
Provided by the Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news in MIT research, innovation and education.
Quote: New system can increase or decrease the expression of synthetic genes (October 13, 2025) retrieved October 13, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.