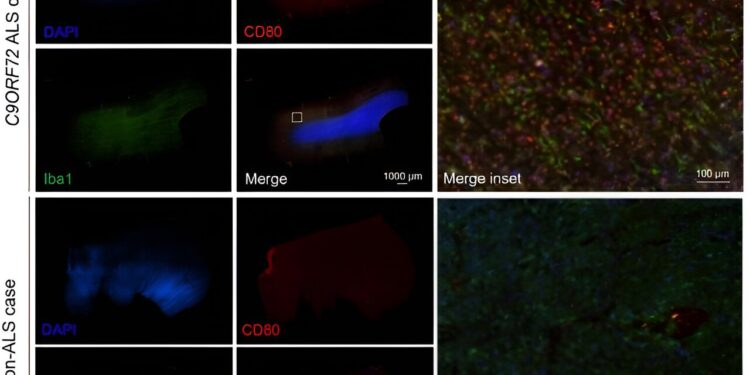
Representative human spinal cord tissue imaged at a 4X objective for DAPI (blue), CD80 (red), and Iba1 (green) from a C9ORF72 ALS case and a non-ALS control. Scale bars represent 1000 µm or 100 µm as shown. Credit: Scientific translational medicine (2024). DOI: 10.1126/scitranslmed.adg7895
Researchers at Case Western Reserve University School of Medicine have discovered why a gene, when mutated, is a common cause of two debilitating brain diseases: amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). .
The study found that the protein generated by this mutant gene, C9ORF72, influences the immune system by regulating the production of interleukin 17A (IL-17A), a potent inflammatory molecule.
ALS is a neurodegenerative disease that causes progressive paralysis due to the loss of neurons in the central nervous system. ALS patients often have a pre-existing autoimmune disease and inflammation in the brain that worsens as muscle function declines.
Aaron Burberry, assistant professor of pathology at the School of Medicine and principal investigator of the study, found in mouse models carrying the C9ORF72 mutation, which affects about 10 percent of ALS patients, that brain inflammation decreased and that mobility improved when the IL-17A gene was activated. blocked.
Burberry and his research team also discovered that another molecule found in the gut (CD80) contributes to inflammation in response to elevations of IL-17A in the brain. Their research was recently published in the journal Scientific translational medicine.
“Our research indicates that blocking IL-17A could be rapidly repurposed to treat ALS patients to slow the progression of their disease or potentially prevent ALS from developing,” Burberry said.
Treatments that block IL-17A have already been approved by the U.S. Food and Drug Administration to treat autoimmune diseases, such as psoriasis and rheumatoid arthritis. These comparable therapies could help ALS patients stop or perhaps reverse disease progression.
“For people living with neurodegenerative disease,” Burberry said, “our work offers hope for a future where quality of life and cognition can be maintained long after their diagnosis.”
Burberry will next study the mechanisms by which C9ORF72 inhibits IL-17A in lymphoid cells and identify elements of the gut microbiome that cause inflammation in the brain.
More information:
Francesco Limone et al, Myeloid and lymphoid expression of C9orf72 regulates IL-17A signaling in mice, Scientific translational medicine (2024). DOI: 10.1126/scitranslmed.adg7895
Provided by Case Western Reserve University
Quote: New study identifies gene suspected of causing ALS and dementia (February 6, 2024) retrieved February 6, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.