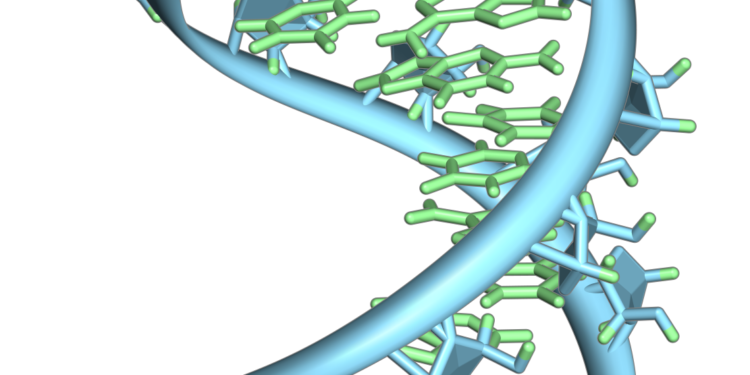
A hairpin loop from a pre-mRNA. The nucleobases (green) and the ribose-phosphate backbone (blue) are highlighted. Note that this is a single strand of RNA that folds back on itself. Credit: Vossman/Wikipedia
Alzheimer’s disease, which is expected to affect approximately 6.7 million patients in the United States in 2023, causes substantial loss of brain cells. But the events that cause neuron death are poorly understood.
A new study from Northwestern Medicine shows that RNA interference may play a key role in Alzheimer’s disease. For the first time, scientists have identified short, toxic RNA strands that contribute to brain cell death and DNA damage in Alzheimer’s and older adult brains. Protective short RNA strands decline with aging, scientists report, which could allow the development of Alzheimer’s disease.
The study also found that older people with greater memory capacity (called SuperAgers) have higher amounts of protective short RNA strands in their brain cells. SuperAgers are individuals aged 80 and over with the memory capacity of individuals 20 to 30 years younger.
“No one has ever linked RNA activities to Alzheimer’s disease,” said the study’s corresponding author, Marcus Peter, the Tom D. Spies Professor of Cancer Metabolism at the Feinberg School of Medicine. Northwestern University. “We found that in aging brain cells, the balance between toxic and protective RNAs shifts toward toxic RNAs.”
The article is published in Natural communications.
Northwestern’s discovery may have relevance beyond Alzheimer’s disease. “Our data provide a new explanation for why, in almost all neurodegenerative diseases, affected individuals live decades without symptoms, then the disease begins to gradually take hold as cells lose protection with age “Peter said.
New treatment route
The findings also suggest a new way to treat Alzheimer’s disease and potentially other neurodegenerative diseases.
Alzheimer’s disease is characterized by the progressive appearance of beta-amyloid plaques, tau neurofibrillary tangles, scarring, and ultimately brain cell death.
“Massive investment in Alzheimer’s drug discovery has focused on two mechanisms: reducing amyloid plaque burden in the brain – which is the hallmark of Alzheimer’s disease diagnosis and accounts for 70 to 80% of the effort – and preventing tau phosphorylation or tangles,” Peter said. “However, treatments aimed at reducing amyloid plaques have not yet resulted in an effective and well-tolerated treatment.
“Our data support the idea that stabilizing or increasing the amount of protective short RNAs in the brain could provide an entirely new approach to stopping or delaying Alzheimer’s disease or neurodegeneration in general.”
Such drugs exist, Peter said, but they would need to be tested in animal models and improved.
The next step in Peter’s research is to determine in different animal and cellular models (as well as in the brains of patients with Alzheimer’s disease) the exact contribution of toxic RNAs to the cell death observed in the disease and to search for best compounds that would selectively increase the level. protective RNAs or block the action of toxic RNAs.
What are toxic and protective short RNAs?
All of our genetic information is stored as DNA in the nucleus of every cell. To turn this genetic information into the building blocks of life, DNA must be converted into RNA which is used by the cellular machinery to produce proteins. RNA is essential for most biological functions.
In addition to these long coding RNAs, there are a large number of short RNAs (sRNAs), which do not code for proteins. They have other critical functions in the cell. One class of these RNAs suppresses long coding RNAs through a process called RNA interference that results in the deactivation of the proteins that the long RNAs code for.
Peter and his colleagues have now identified very short sequences present in some of these sRNAs which, when present, can kill cells by blocking the production of proteins necessary for cell survival, thereby leading to cell death. Their data suggest that these toxic RNAs are involved in the death of neurons, thus contributing to the development of Alzheimer’s disease.
Toxic RNAs are normally inhibited by protective RNAs. One type of sRNA is called microRNA. Although microRNAs play several important regulatory roles in cells, they are also the primary protective sRNA species. They are the equivalent of guards that prevent toxic RNAs from entering the cellular machinery that executes RNA interference. But the number of guards decreases with aging, allowing toxic RNAs to damage cells.
Scientists analyzed brains from mouse models of Alzheimer’s disease, brains from young and old mice, induced neurons derived from pluripotent stem cells from normal individuals (young and old) and patients with the disease Alzheimer’s disease, the brains of a group of individuals aged over 80 with memory capacity equivalent to that of individuals aged 50 to 60 and multiple neuron-like cell lines derived from the human brain and treated with beta-amyloid fragments, a trigger for Alzheimer’s disease.
More information:
Death induced by deletion of the survival gene (DISE) is correlated with neurotoxicity in Alzheimer’s disease and aging, Natural communications (2024).
Provided by Northwestern University
Quote: New study finds that short, toxic RNAs kill brain cells and could enable the development of Alzheimer’s disease (January 18, 2024) retrieved January 18, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.