Graphical summary. Credit: Cell Reports Medicine (2024). DOI: 10.1016/j.xcrm.2024.101421
In the treatment of aggressive lymphomas and blood cancer (leukemia), chimeric antigen receptor T cells (CAR T cells) are increasingly used. For this therapy, immune cells are taken from patients and programmed by genetic engineering to detect proteins in malignant tumor cells.
Back in the body, the CAR T cells then fight cancer cells. Due to some significant side effects, this therapy requires extreme caution and long hospital stays. Scientists at the University Hospital Cologne are therefore investigating new mechanisms to make CAR T cell-based immunotherapy more effective and safer.
The team led by Dr. Markus Chmielewski from the Cologne Center for Molecular Medicine (CMMC) now presents a new strategy to make CAR T cell-based immunotherapy more effective and safer. The study titled “An anti-CD19/CTLA-4 switch enhances the efficacy and selectivity of CAR T cells targeting DLBCLs upregulated by CD80/86” was published in the journal Cell Reports Medicine.
From bedside to bench
This strategy is based on the examination of tissues from lymphoma patients treated in Department I of Internal Medicine at the University Hospital Cologne. The research team discovered an increasing number of CD80 and CD86 surface proteins in tumor cells.
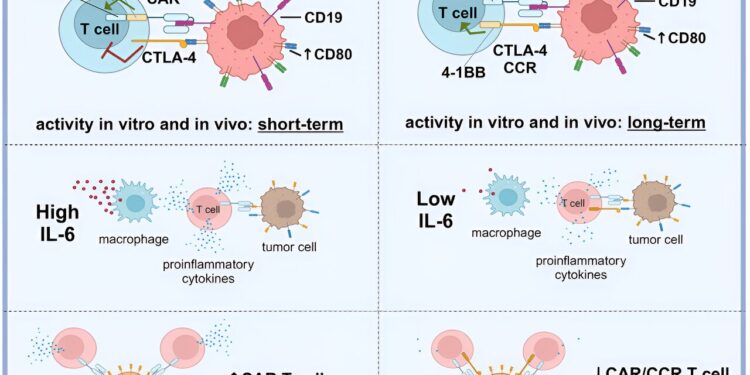
Such high numbers of these proteins are not found in healthy B lymphocytes (B cells), the affected cells of the immune system in lymphomas. Unlike previously available CAR T cell therapies, which typically target only the surface protein CD19, the researchers used two CAR constructs with different target proteins that complement each other to activate T cells against tumor cells. CD19 was selected as the target of the first CAR construct because it is present in all B lymphocytes.
Another target is CD80/CD86, present on malignant B lymphocytes. To this end, the researchers used a binding domain, a protein sequence capable of recognizing and binding both CD80 and CD86 in the form of a locking principle.
The two CAR constructs work together as an “AND” switch that only allows the CAR T cell to fully activate and fight the target cell if both surface markers are detected. This does not harm normal B cells that only have the CD19 marker, which is the case with CAR T cell therapies approved to date. This allows normal B cells to continue their important work as an integral part of the immune system.
This also works the other way: if only the second CAR construct binds to CD80 or CD86, but there is no CD19 binding.
“Our CAR T cells exhibit more differentiated and longer-lasting stimulation through the biological “AND” switch. They fight cancer cells more effectively than previously approved CAR T cell approaches and, at the same time, do not harm healthy B cells and to other CD19-positive cells,” said Fabian Prinz, lead author of the study and a medical student in his clinical rotation, summarizing the results.
The results were obtained in the laboratory using cell cultures as well as mouse models. “Our next steps for the coming years are clear: preparing a clinical trial and testing the proposed strategy in patients with B-cell lymphoma,” Chmielewski said.
“The preclinical success of our CAR T cell approach is an example of the importance of translational research that recognizes real-world patient problems, translates them into scientific problems that can be solved in the laboratory, and finds solutions through experiments .”
More information:
Lars Fabian Prinz et al, An anti-CD19/CTLA-4 switch enhances the efficacy and selectivity of CAR T cells targeting DLBCL upregulated by CD80/86, Cell Reports Medicine (2024). DOI: 10.1016/j.xcrm.2024.101421
Provided by the University of Cologne
Quote: New strategy for safer CAR T cell therapy in lymphomas (February 9, 2024) retrieved February 9, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.