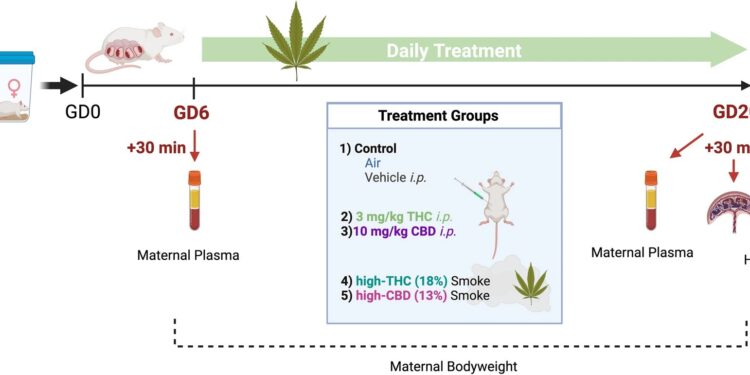
Experimental timeline of gestational cannabis exposure. Rats were raised and treated once daily between gestational day (GD) 6 and 20 for 15 consecutive days of treatment. Maternal and fetal tissues were harvested 30 min after the first (GD6) and last (GD20) treatment. THC, CBD, and their metabolites were quantified in maternal plasma by high-performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS). Protein samples from the placenta and whole fetal brain were prepared for quantification of cytokines and chemokines. Created with Biorender.com. Credit: Scientific reports (2023). DOI: 10.1038/s41598-023-47861-8
The legalization of cannabis in Canada is driving demand for scientifically supported information to inform public health messages.
Until recently, preclinical cannabis research – conducted without the use of human participants to determine preliminary information such as the substances’ safety and toxicity – largely involved injecting cannabinoids into rodent models.
To develop a preclinical rodent model that more accurately reflects cannabis use in Canada, USask researchers Drs. John Howland (Ph.D.) and Robert Laprairie (Ph.D.), along with their graduate students Tallan Black and Ilne Barnard, conducted studies using a new cannabis smoke delivery system by burning varieties available in the trade.
“You can buy the strains we used in stores as a consumer in Canada,” said Howland, a professor in the Faculty of Medicine’s department of anatomy, physiology and pharmacology. “Canadian scientists can now easily use these products in their research.”
The smoke chambers used in their research are the first of their kind in Canada to be adapted to burn cannabis buds, replacing the conventional use of injected cannabinoids.
This generates physiological and pharmacological data that provides a more accurate window into the human experience. Using smoke inhalation – the most common method of administration among Canadian users of high-THC cannabis – and cannabis products available at local dispensaries, the findings draw closer parallels to physiological and molecular functions of human consumption and shed light on important next steps in the team’s work.
“Preclinical models give us insight,” said Barnard, a Ph.D. student in the Department of Anatomy, Physiology and Pharmacology. “Having a preclinical model to understand what happens in the brain (after cannabis use) can also inform clinical research.”
Approaching the topic from a basic science and public health perspective, this research takes an interdisciplinary approach to address an urgent public health debate regarding the safe use of cannabis.
Using the team’s smoke exposure method, Barnard modeled and assessed the impact of acute exposure to high-THC cannabis on young adults’ working memory function. The results were published in in Euro end of 2023.
“We were able to observe a more nuanced behavioral effect following exposure to cannabis,” she said. “What we found is that following acute cannabis exposure, there is an increased deficit in working memory when the task is more difficult.”
Working memory plays an important role in daily functioning and in human disorders such as schizophrenia, and will be evaluated further in the team’s future research.
Black, who holds a Ph.D. A USask College of Pharmacy and Nutrition and College of Medicine student modeled the impact of repeated exposure to cannabis in pregnant rodents, comparing the effects of smoke exposure to those of an injection and between strains rich in CBD and THC.
“This model of in utero smoke exposure is the first of its kind,” she said. “It’s critical because the understanding of the effects of exposure to cannabis smoke in utero is so new…we just don’t have the information.”
Recently published in Scientific reports, Black’s findings confirm notable physiological differences after exposure to smoke, compared to the use of injected cannabinoids. As a result, these findings validate the importance of using smoke in future research regarding the impacts of cannabis use during pregnancy.
As this research continues to unfold, Black cautions that “in a country where cannabis is legalized, it’s really important to take it slow and realize that we just don’t know (the full impacts of cannabis). exposure to cannabis).
After validating the smoke exposure model and identifying preliminary findings, the team aims to continue to better understand the functions and risks of cannabis use in Canada.
This includes the study of behaviors most susceptible to disruption following exposure to cannabis, the behavioral outcomes to monitor in children of a mother who used cannabis during pregnancy and the impact of different experiences (positive or negative ) following exposure to cannabis.
Ultimately, the team aims to improve and refine public health messaging to benefit Canadian cannabis consumers.
“We believe that further education of the general public regarding cannabis will allow individuals to make informed decisions regarding their use and the type of cannabis they can consume,” Howland said. “These studies could also lead to the development of evidence-based interventions to mitigate the negative effects of cannabis use.”
More information:
L. Barnard et al, High THCC cannabis smoke impairs incidental memory capacity during spontaneous tests of novelty preference for objects and odors in male rats, in Euro (2023). DOI: 10.1523/ENEURO.0115-23.2023
Tallan Black et al, Characterization of plasma cannabinoid concentration, maternal health and cytokine levels in a rat model of prenatal cannabis smoke exposure, Scientific reports (2023). DOI: 10.1038/s41598-023-47861-8 Ilné
Provided by the University of Saskatchewan
Quote: New smoke exposure model could help more accurately determine effects of cannabis on consumers (January 11, 2024) retrieved January 11, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.