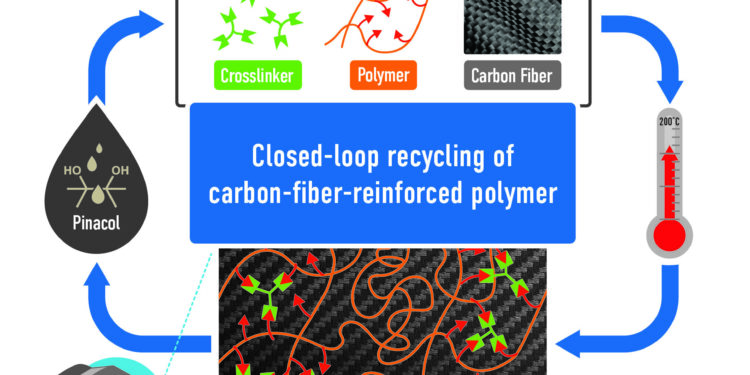
A polymer, functionalized carbon fibers and a cross-linking agent are mixed and cured. The components can be recovered by adding an alcohol, pinacol. Credit: Philip Gray and Anisur Rahman/ORNL, US Department of Energy
In a victory for chemistry, inventors at the Department of Energy’s Oak Ridge National Laboratory have designed a closed-loop pathway to synthesize an exceptionally strong carbon fiber-reinforced polymer, or CFRP, and then recover all its materials firsts.
A lightweight, strong and durable composite material, CFRP is useful in reducing weight and increasing fuel efficiency in automobiles, aircraft and spacecraft. However, conventional CFRPs are difficult to recycle. Most are single-use materials, so their carbon footprint is significant. In contrast, ORNL’s closed-loop technology, published in Cellular Reports Physical Sciencesaccelerates the resolution of this great challenge.
“We incorporated dynamic cross-linking into a base polymer to functionalize it. Then we added a cross-linker to make it similar to thermoset materials,” said ORNL chemist and inventor Md Anisur Rahman. “Dynamic cross-linking allows us to break chemical bonds and reprocess or recycle carbon fiber composite materials.”
A typical thermoset material is permanently crosslinked. Once synthesized, hardened, molded and shaped, it cannot be reprocessed. ORNL’s system, on the other hand, adds dynamic chemical groups to the polymer matrix and its embedded carbon fibers. The polymer matrix and carbon fibers can undergo multiple reprocessing cycles without loss of mechanical properties, such as strength and toughness.
Rahman led the study with ORNL chemist Tomonori Saito, who was honored by Battelle in 2023 as ORNL Inventor of the Year. Rahman and ORNL postdoctoral fellow Menisha Karunarathna Koralalage conducted most of the experiments. The trio has filed a patent application for this innovation.
“We invented a durable and recyclable carbon fiber composite,” Saito said. “The fiber and the polymer have very strong interfacial adhesion thanks to the presence of dynamic bonds.” The interface locks materials together via covalent interactions and unlocks them on demand using heat or chemistry. Saito added: “The functionalized fiber has dynamic exchangeable cross-linking with this polymer. The composite structure is really strong because of the interface characteristics. This makes it a very, very strong material.”
Conventional polymers like thermoset epoxies are typically used to permanently bond materials such as metal, carbon, concrete, glass, ceramics and plastic to form multi-component materials such as composites. However, in the ORNL material, the polymer, carbon fibers and cross-linking agent, once thermoset, can be reincarnated into these starting materials. Components of the material can be recycled when a special alcohol called pinacol replaces the covalent bonds of the cross-linking agent.
Closed-loop recycling at laboratory scale results in no loss of raw materials. “When we recycle composites, we recover 100% of the raw materials: the cross-linking agent, the polymer, the fiber,” Rahman said.
“This is the importance of our work,” Saito said. “Other composite recycling technologies tend to lose component raw materials during the recycling process.”
Other advantages of reversibly cross-linked CFRPs are rapid thermosetting behavior, self-adhesive behavior and repair of microcracks in the composite matrix.
In the future, closed-loop recycling of CFRPs could transform low-carbon manufacturing as circular lightweight materials are integrated into clean energy technologies.
The researchers took inspiration from nature, which uses dynamic interfaces to create robust materials. Mother-of-pearl, the iridescent mother-of-pearl contained in the shells of marine mussels and other molluscs, is exceptionally resistant: it can deform without breaking. Additionally, marine mussels adhere strongly to surfaces but dissipate energy to release them when necessary.
The researchers aimed to optimize the interfacial chemistry between the carbon fibers and the polymer matrix to strengthen interfacial adhesion and improve the toughness of CFRP. “The strength of our composite is almost twice that of a conventional epoxy composite,” Rahman said. “Other mechanical properties are also very good.”
The tensile strength, or the stress a material can withstand when pulled, was the highest ever reported among similar fiber-reinforced composite materials. It was 731 megapascals, stronger than stainless steel and a conventional automotive epoxy-based CFRP composite.
In the ORNL material, the dynamic covalent bond between the fiber interface and the polymer exhibited 43% higher interfacial adhesion than polymers without dynamic bonds.
Dynamic covalent bonds enable closed-loop recycling. In a typical matrix material, the carbon fibers are difficult to separate from the polymer. ORNL’s chemical method, which cuts fibers at functional sites, separates the fibers from the polymer for reuse.
Karunarathna Koralalage, Rahman and Saito modified a base polymer, called S-Bpin, with help from Natasha Ghezawi, a graduate student at the Bredesen Center for Interdisciplinary Research and Graduate Education at the University of Tennessee in Knoxville. They created a recycled styrene-ethylene-butylene-styrene copolymer, which incorporates boronic ester groups that bond covalently with a cross-linker and fibers to generate the strong CFRP.
As CFRP is a complex material, its detailed characterization required diverse expertise and instrumentation. ORNL’s Chris Bowland tested the tensile properties. Using Raman mapping, ORNL’s Guang Yang showed the distribution of chemical and structural species. Catalin Gainaru and Sungjin Kim, both of ORNL, captured rheological data, and Alexei Sokolov, UT-ORNL Governor’s Chair, elucidated them.
Scanning electron microscopy by Bingrui Li, of ORNL and UT, revealed that the carbon fiber retained its quality after recycling. Vivek Chawla and Dayakar Penumadu, both from UT, analyzed the interlaminar shear strength. Using X-ray photoelectron spectroscopy, ORNL’s Harry Meyer III confirmed which molecules were attached to the fiber surfaces. ORNL’s Amit Naskar, a renowned carbon fiber expert, reviewed the paper.
Scientists have discovered that the degree of dynamic crosslinking is important. “We found that 5 percent cross-linking works better than 50 percent,” Rahman said. “If we increase the amount of cross-linker, it starts to make the polymer brittle. This is because our cross-linker has three bulky, hand-like structures that are capable of making more connections and decreasing the flexibility of the polymer .”
Next, the research team wants to conduct similar studies with fiberglass composites, which maintain high performance while reducing the cost and carbon footprint of applications in aerospace, automotive, marine, sport, construction and engineering. They also hope to reduce the costs of the new technology to optimize the commercial prospects of a future licensee.
“This step will pave the way for more applications, including wind turbines, electric vehicles, aerospace materials and even sporting goods,” Rahman said.
More information:
Md Anisur Rahman et al, Tough and Recyclable Carbon Fiber Composites with Exceptional Interfacial Adhesion via Custom Vitrimer Fiber Interface, Cellular Reports Physical Sciences (2023). DOI: 10.1016/j.xcrp.2023.101695
Provided by Oak Ridge National Laboratory
Quote: New process enables complete recovery of raw materials from strong polymer composites (February 8, 2024) retrieved February 8, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.