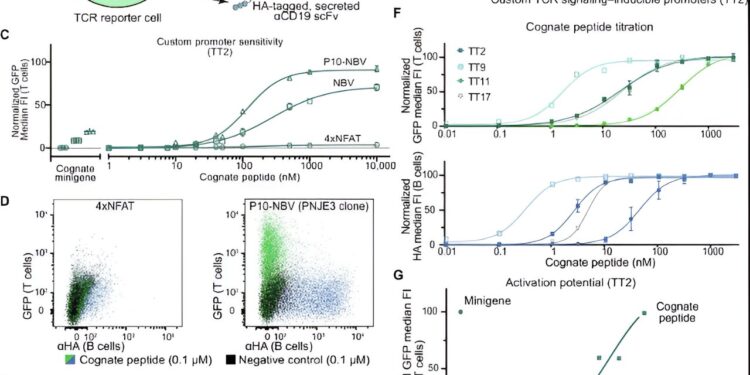
Development and comparative analysis of a TCR activation reporting system. (A) Schematic illustrating the construction of a TCR reporter cell line (left) transgenically modified with an inducible promoter and a TCR of interest. Upon interaction of a cognate ligand, the reporter cell produces cytoplasmic GFP and a secreted HA-tagged antibody fragment specific for CD19, a B cell-specific surface protein. B cells (right) used as APCs are transduced with a minigene-containing epitope or exogenously loaded with peptide epitopes. Upon activation of reporter T cells, B cells are marked by secreted αCD19 scFv. (B) Median GFP (green) and αHA (blue) fluorescent signals upon interaction of a cognate ligand for TT2 transgenic Jurkat cells with tailor-made inducible TCR signaling promoters. Negative control, unrelated interaction (from the P10-NBV promoter as an example). (C) Minigene and peptide dilution curves of a cognate epitope for P10-NBV, NBV, and 4xNFAT transposon-integrated inducible reporter cassettes (bulk T cell population before single clone selection). (D) Superimposed dot plots depicting GFP expression of T cells (y-axis, green) and anti-CD19 scFv labeling of B cells (x-axis, blue) in a single related target coculture. Black dots, GFP fluorescence in unrelated control coculture. Left: 4xNFAT promoter. Right: single PNJE3 transgenic clone of the P10-NBV promoter. (E) TCR-independent titration curves for two reference TCRs (TT7 and TT11) using an αCD3 antibody (OKT-3). (F) Titration curves of PNJE3 activation of four reference TCRs (TT2, TT9, TT11, and TT17) with related peptide epitopes of the tetanospasmin protein of Clostridium tetani. Upper graph, T cell GFP expression; Lower plot, anti-CD19 scFv labeling of B cells. Error bars indicate SD from three replicates. (G) Activation of PNJE3 in coculture with BOLETH cells following electropulsion of a cognate peptide and full-length tetanus toxoid protein, or transduction with a 400 amino acid epitope-containing minigene. Representative data from two independent titration experiments. Credit: Scientists progress (2024). DOI: 10.1126/sciadv.adk3060
BioMed Scientists progress. The primary goal of the collaboration was to understand the role of specific T cell responses in a patient with an aggressive subtype of diffuse glioma who experienced sustained remission after receiving a neoepitope peptide vaccine at University Hospital from Mannheim.
The work is based on a collaboration between the two institutions and researchers from the German Cancer Research Center (DKFZ), the University of Heidelberg and the Helmholtz Institute for Translational Oncology (HI-TRON).
T cells are essential for maintaining human health by eliminating tumor cells from the body, a process that is driven by the specific interaction of unique receptors on the surface of T cells (T cell receptors or TCRs) with mutant peptides (antigens) on the surface. of tumor cells. Identification of these cancer-specific antigens and the TCRs that bind them is the basis of current efforts to develop targeted cancer therapies. Until now, functionally robust high-throughput approaches to address this challenge have been lacking.
Dr. John M. Lindner and his research team at the BioMed X Institute in Heidelberg designed the T-FINDER platform to solve this problem. The platform is capable of rapidly screening thousands of potential interactions between TCRs and antigens on the surface of potential target cells to determine their ability to activate T cells.
Led by a trio of senior scientists (Miray Cetin, Dr Veronica Pinamonti and Dr Theresa Schmid), the team first generated a highly sensitive reporter cell line for T cell activation. This reporter is at the heart of T- FINDER, which can sensitively read the specificity of a number of T cell-activating receptors (e.g., CAR-T receptors in addition to classical TCRs) and ligands.
For the latter, Dr. Pinamonti provided solutions to key aspects enabling the detection of peptides presented by HLA class II during his Ph.D. thesis work, leading to the development of new strategies to stimulate the processing and presentation of antigens by the target cell. This class of ligands has until now been very difficult to study experimentally and, with its superior sensitivity, forms the basis of T-FINDER’s advantages in this area.
In an accompanying manuscript, the team collaborated with a research team led by Dr. Ed Green from the Neuroimmunology and Brain Tumor Immunology Clinical Cooperation Unit (head: Professor Michael Platten) of the DKFZ and the hospital University of Mannheim. Researchers applied T-FINDER to decode the immune response of two diffuse midline glioma patients vaccinated against the H3 mutation that caused their cancer.
Patients receiving the H3 vaccine showed promising but heterogeneous results, and with the help of T-FINDER, both groups were able to accurately map the functional immune response of a patient in remission to TCRs binding HLA-presented epitopes class II of the H3 mutant. This work provides key insights into the mechanism of anti-tumor T cell responses in these patients and will support ongoing vaccination studies.
“We are excited to publish the results of our collaboration with Ed Green, Michael Platten and their colleagues,” said John Lindner, head of immunology discovery at the BioMed X Institute. “Our joint research demonstrated sensitivity, the unique flexibility and overall performance of our new T-FINDER platform, especially for targets presented by HLA class II, which have always been a challenge in the past.
“Previously, we were limited in the tools we could use to study epitopes presented by class II, such as mutant H3. The T-FINDER platform allowed us to identify and compare dozens of H3-reactive TCRs, allowing us to track patient responses. for vaccination today, as well as reference TCRs for autologous cell therapies of tomorrow,” said Ed Green, head of the ImmunoGenomics team in Michael Platten’s laboratory at DKFZ.
More information:
Miray Cetin et al, T-FINDER: a highly sensitive pan-HLA platform for the discovery of functional T cell receptors and ligands, Scientists progress (2024). DOI: 10.1126/sciadv.adk3060
Tamara Boschert et al, Vaccination against the H3K27M neoepitope in diffuse midline glioma induces B and T cell responses in various HLA loci of a cured patient, Scientists progress (2024). DOI: 10.1126/sciadv.adi9091
Provided by University Hospital Mannheim
Quote: New platform provides deep insights into T cell responses against a new cancer vaccine (February 6, 2024) retrieved February 6, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.