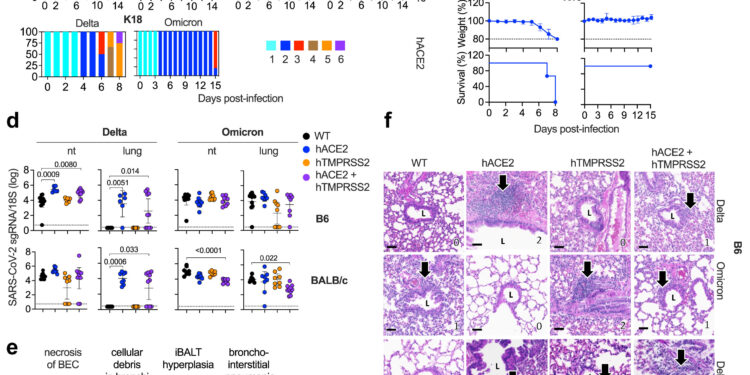
SARS-CoV-2 delta and omicron BA.1 infectivity, clinical disease, and lung pathology in hACE2 and hTMPRSS2 single and double KI mice. Credit: eBioMedicine (2024). DOI: 10.1016/j.ebiom.2024.105361
Scientists at the La Jolla Institute of Immunology (LJI) have developed six humanized mouse lines that can serve as valuable models for studying human cases of COVID-19.
According to their new study in eBioMedicineThese mouse models are important for COVID-19 research because their cells were engineered to include two important human molecules involved in SARS-CoV-2 infection of human cells – and these humanized mice were generated on two different immunological environments. The new models can help shed light on how SARS-CoV-2 moves through the body and why different people experience wildly different symptoms of COVID-19.
“With these mouse models, we can model epidemiologically relevant SARS-CoV-2 infection and vaccination contexts, and we can study all relevant tissues (not just blood) at different times after infection and /or vaccination,” explains Professor Sujan of LJI. Shresta, Ph.D., who co-led the research with LJI Senior Director of Histopathology Kenneth Kim, Dipl. ACVP, and the late Kurt Jarnagin, Ph.D., of Synbal, Inc.
Already, these new mouse models have helped scientists get a clearer picture of how SARS-CoV-2 affects humans. They are also accessible to the entire COVID-19 research community.
“This work is part of LJI’s mission to contribute to pandemic preparedness around the world,” Shresta says.
Mouse models are an essential tool for understanding infection
Shresta’s lab is known for producing mouse models to study immune responses to infectious diseases such as the dengue virus and the Zika virus. In 2021, his laboratory partnered with Synbal, Inc., a preclinical biotechnology company based in San Diego, California, to develop multigene humanized mouse models for COVID-19 research.
Shresta and Jarnagin collaborated to produce mice that express either human ACE2, human TMPRSS2, or both molecules in the genetic backgrounds of C57BL/6 and BALB/c mice. “Immunologists have discovered that these two genetic backgrounds in mice elicit different immune responses,” explains Shresta.
As Shresta explains, having the ability to include genes for one or both of these molecules in two different mouse genetic backgrounds gives scientists the opportunity to study two key areas. First, they can examine how each of these molecules contributes to infection with different variants of SARS-CoV-2. Second, they can study how the host’s genetic background might influence disease progression and immune response following infection with different variants.
Focus on infected tissues
The researchers then took a closer look at how these models responded to real SARS-CoV-2 infection. Shailendra Verma, Ph.D., LJI postdoctoral fellow, worked in LJI’s High Containment Facility (BSL-3) to collect tissue samples from the various strains of mice exposed to SARS-CoV-2.
“This work would not have been possible if we did not have a BSL-3 facility at LJI,” says Shresta, who worked closely with LJI’s Environmental Health and Safety Department to conduct several cutting-edge studies at the facility.
Then, Kim, a board-certified pathologist, examined the tissue samples and compared them to pathology results from humans with COVID-19.
Kim’s analysis showed signs of SARS-CoV-2 infection in the lungs, which are also the tissue most vulnerable to SARS-CoV-2 infection in humans. Kim could also see mouse immune cells respond to infection in a way that mirrored the human immune response.
By characterizing these responses in the new mouse models, researchers have established a foundation for understanding the immune heterogeneity – or the broad range of immune responses – of SARS-CoV-2-induced disease.
“There is no perfect animal model, but our goal is always to create an animal model that recapitulates human disease and immune response as much as possible,” says Shresta.
The new mouse models could prove useful for studying responses to emerging SARS-CoV-2 variants and future coronaviruses with pandemic potential.
“Not only are these models useful for current COVID-19 studies, but should there be another coronavirus pandemic – with a virus that uses the same ACE2 receptor and/or TMPRSS2 molecule for entry viral in human cells – then these mouse lines on two different genetic backgrounds will be ready,” says Kim.
Other study authors include Erin Maule, Paolla BA Pinto, Chris Conner, Kristen Valentine, Dale O Cowley, Robyn Miller, Annie Elong Ngono, Linda Tran, Krithik Varghese, Rúbens Prince dos Santos Alves, Kathryn M. Hastie and Erica Ollmann Saphire. .
More information:
Shailendra Kumar Verma et al, Influence of Th1 versus Th2 immune bias on viral, pathological and immunological dynamics in human ACE2 knock-in mice infected with the SARS-CoV-2 variant, eBioMedicine (2024). DOI: 10.1016/j.ebiom.2024.105361
Provided by La Jolla Institute for Immunology
Quote: New mouse models provide valuable window into COVID-19 infection (October 1, 2024) retrieved October 1, 2024 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.