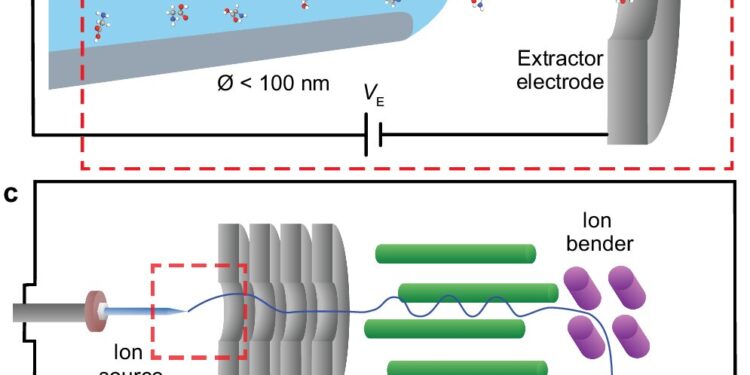
Comparison of a conventional electrospray ion source with a nanopore ion source. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-51455-x
Mass spectrometry is a powerful technique that allows scientists to break down and identify the building blocks of almost anything by measuring the mass of the tiny particles that make it up. However, it has one major limitation: about 99% of the sample measured is typically lost before analysis can even begin.
This loss rate limits the technology’s potential. It reduces accuracy and sensitivity, wastes resources, and complicates sample preparation, which can lead to additional errors. But that may not be the case for much longer.
A research team at Brown University has developed a new method of transferring ions analyzed by mass spectrometers, dramatically reducing sample loss so that nearly all of it remains intact.
“The conventional technique for producing ions for mass spectrometry, called electrospray ionization, basically involves placing a very sharp needle right in front of the mass spectrometer, hitting it with an electric field that extracts a jet of charged droplets that eventually dry out to produce bare ions that enter the mass spectrometer in open air,” said Nicholas Drachman, a doctoral student in physics at Brown who led the work.
“Basically, it’s a process where you spray your sample all over to produce these ions and only introduce a tiny fraction of them into the vacuum of the mass spectrometer for analysis. Our approach avoids all of that.”
Called a nanopore ion source, the breakthrough overcomes a long-standing bottleneck in science and has the potential to revolutionize mass spectrometry technology. The Brown team describes the groundbreaking innovation in Nature Communications.
The key lies in a tiny capillary the researchers developed, whose opening is about 30 nanometers wide, about 1,000 times smaller than the width of a human hair. For comparison, the conventional needle used in electrospray has an opening about 20 micrometers wide, about 600 times larger than the tube developed at Brown.
The new nanotube also has the unique ability to transfer ions dissolved in water directly into the vacuum of a mass spectrometer, rather than producing a jet of droplets that must be dried to access the ions.
Additionally, conventional mass spectrometers typically suck up a significant amount of gas along with the ions during the process, requiring multiple stages of vacuum pumps to suck up the ions. This new advancement means the gas won’t need to be pumped out because it won’t be sucked up, the researchers say.
“Rather than putting it in front of a mass spectrometer and generating this spray of droplets, we put it directly into the mass spectrometer, avoiding this complicated process of spraying, drying and putting it in a vacuum,” Drachman said. “By generating ions directly in a vacuum, it dramatically reduces the pumping requirements, which should greatly simplify the complex hardware of mass spectrometers.”
The Brown team was inspired by nanopore sequencing in DNA and plans to commercialize their idea for widespread use by protein researchers, including the long-sought goal of sequencing proteins one amino acid at a time.
“Mass spectrometry is the best way to look at proteins, which are made up of amino acids with all sorts of different chemical and physical properties, because you can tell them apart by the mass of their ions with great certainty,” said Derek Stein, a professor of physics at Brown and an author of the paper.
“Proteomics has not seen the same advances as genomics in the last two decades, and so there has been this hunger for a technology that can improve protein analysis. By eliminating this problem of sample loss, it should allow much more sensitive analyses, such as sequencing the amino acids of a protein molecule one by one and in sequential order. That’s the genius idea that motivated our work.”
The team has spent the last 10 years working on this new method. They began by custom-designing their own mass spectrometer that could house the single ion source in a vacuum, unlike traditional designs where the ion source is separate from the instrument and sits in open air.
The team built the key component of their transfer device by using a special machine to heat a glass tube in the middle, then gently pull it apart to create an extremely small opening at the end invisible to the naked eye.
Trial and error played a big part in the process, often leading to weeks of frustration as they struggled to get everything to work coherently at the end of the capillary, which is far too small to inspect with the naked eye.
“Some weeks we didn’t know if we were cursed by God himself or something. Things stopped working,” Stein said. “Other weeks, everything worked perfectly.”
The team’s persistence paid off. They were able to demonstrate that ion analysis with their new transfer method matches detections using traditional methods, but with much less sample loss, providing a more efficient and accurate way to analyze tiny particles.
“We had to convince people in the proteomics field that we could generate the same kind of ions that they were used to by conventional electrospray, and that we could do it in this different and, we thought, better way,” Drachman said.
The analysis described in the paper serves as a proof of concept for the method. The researchers then want to exploit the full potential of their nanopore ion source.
“We need to show that this can improve the workflow of proteomic analyses,” Drachman said. “We would like to take this further and make it something that will improve the science for researchers across the board.”
More information:
Nicholas Drachman et al, Nanopore ion sources deliver individual amino acid and peptide ions directly into high vacuum, Nature Communications (2024). DOI: 10.1038/s41467-024-51455-x
Provided by Brown University
Quote:New mass spectrometry technology could transform analysis of tiny samples (2024, September 9) retrieved September 9, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.