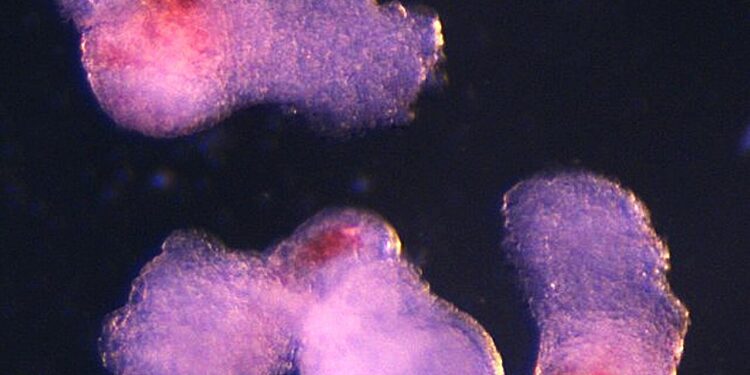
The team observed the emergence of three-dimensional structures resembling embryos under a microscope in the laboratory. These began producing blood (seen here in red) after about two weeks of development, mimicking the process of development in human embryos. Credit: Jitesh Neupane, University of Cambridge
Scientists at the University of Cambridge have used human stem cells to create three-dimensional embryo-like structures that replicate aspects of very early human development, including the production of blood stem cells. The results are published in the journal Cell Reports.
Human blood stem cells, also known as hematopoietic stem cells, are immature cells that can develop into any type of blood cell, including red blood cells that carry oxygen and various types of white blood cells essential for the immune system.
The embryo-like structures, which scientists have named “hematoid,” self-organize and begin producing blood after about two weeks of development in the laboratory, mimicking the developmental process of human embryos.
The structures differ from real human embryos in many ways and cannot develop in them because they lack several embryonic tissues, as well as the yolk sac and placenta necessary for their further development.
Hematoids hold exciting potential for better understanding blood formation during early human development, for simulating blood disorders like leukemia, and for producing durable blood stem cells for transplants.
The human stem cells used to derive hematoid cells can be created from any cell in the body. This means that this approach also holds great potential for personalized medicine in the future, by enabling the production of blood that is fully compatible with the patient’s body.
Although there are other methods for generating human blood stem cells in the laboratory, these require a cocktail of additional proteins to support the growth and development of the stem cells. The new method mimics the natural developmental process, based on a model similar to a self-organizing human embryo, in which the cells’ intrinsic supporting environment drives the formation of blood cells and the beating of heart cells within the same system.
Dr Jitesh Neupane, a researcher at the Gurdon Institute at the University of Cambridge and first author of the study, said: “It was an exciting moment when the blood-red color appeared in the dish – it was visible even to the naked eye. »
He added: “Our new model mimics the development of human fetal blood in the laboratory. This sheds light on how blood cells form naturally during human embryogenesis, offering potential medical advances for testing drugs, studying early blood and immune system development, and modeling blood disorders like leukemia.
Professor Azim Surani from the Gurdon Institute at the University of Cambridge, lead author of the paper, said: “This model offers a powerful new way to study blood development in the early human embryo. Although still in its early stages, the ability to produce human blood cells in the laboratory marks an important step toward future regenerative therapies, which use a patient’s own cells to repair and regenerate damaged tissues.
Dr Geraldine Jowett from the Gurdon Institute at the University of Cambridge, co-first author of the study, said: “Haematoid captures the second wave of blood development that can give rise to specialized immune cells or adaptive lymphoid cells, such as T cells, opening exciting avenues for their use in modeling healthy and cancerous blood development. »
The team observed the emergence of three-dimensional hematoid cells under a microscope in the laboratory. After just a few days, these had self-organized into three germ layers – called ectoderm, mesoderm and endoderm – the foundations of the human body plan that are crucial for shaping every organ and tissue, including blood. Credit: Jitesh Neupane, University of Cambridge
Self-organized structures
The new human embryo-like model simulates the cellular changes that occur during the very early stages of human development, when our organs and blood system begin to form.
The team observed the emergence of three-dimensional hematoid cells under a microscope in the laboratory. By the second day, these had self-organized into three germ layers – called ectoderm, mesoderm and endoderm – the foundations of the human body plan that are crucial for shaping every organ and tissue, including blood.
By the eighth day, beating heart cells had formed. These cells eventually give rise to the heart of a developing human embryo.
On day 13, the team saw red spots of blood appear in the hematoid cells. They also developed a method demonstrating that hematoid blood stem cells can differentiate into different types of blood cells, including specialized immune cells, such as T lymphocytes.
Shining a light on early human development
Stem cell-derived embryo models are essential for advancing our knowledge of early human development.
The hematoid blood cells develop to a stage that roughly corresponds to the fourth or fifth week of human embryonic development. This very first stage of life cannot be observed directly on an actual human embryo, because it has then implanted itself in the mother’s womb.
There are clear regulations governing stem cell-based human embryo models, and all research modeling human embryo development must be approved by ethics committees before proceeding. This study received necessary approvals and the resulting paper was peer-reviewed.
The scientists have patented this work through Cambridge Enterprise, the innovation arm of the University of Cambridge, which helps researchers translate their work into world-leading economic and social impact.
More information:
A post-implantation model of human embryo development includes a definitive hematopoietic niche, Cell Reports (2025). DOI: 10.1016/j.celrep.2025.116373. www.cell.com/cell-reports/full… 2211-1247(25)01144-1
Provided by the University of Cambridge
Quote: New laboratory-grown human embryo model produces blood cells (October 13, 2025) retrieved October 13, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.