Graphical summary. Credit: Cell Reports (2024). DOI: 10.1016/j.celrep.2023.113662
A new Cell Reports A paper from Bing Yao’s lab in Emory’s Department of Human Genetics provides insight into the mechanisms underlying several neurodegenerative diseases, such as ALS (amyotrophic lateral sclerosis) and Alzheimer’s disease.
This can be summed up in one line: TDP-43 keeps the genetic zombies at bay.
Yao says zombies are only part of the story, but they represent a window into what the TDP-43 protein does in our cells and what happens when its function ceases and the regulatory machinery of cells fail.
TDP-43 is one of those notoriously aggregation-prone proteins familiar to researchers studying neurodegenerative diseases. It’s like amyloid and tau, but maybe not as famous. Cytoplasmic TDP-43 aggregates are considered the “pathological hallmark” of ALS, and their aggregates can also be found in Alzheimer’s disease brain samples.
“Most studies of TDP-43 function focus on it as an RNA-binding protein,” says Yao. “But our analysis really shows how it plays an epigenetic and genome-wide role, which is not fully appreciated.”
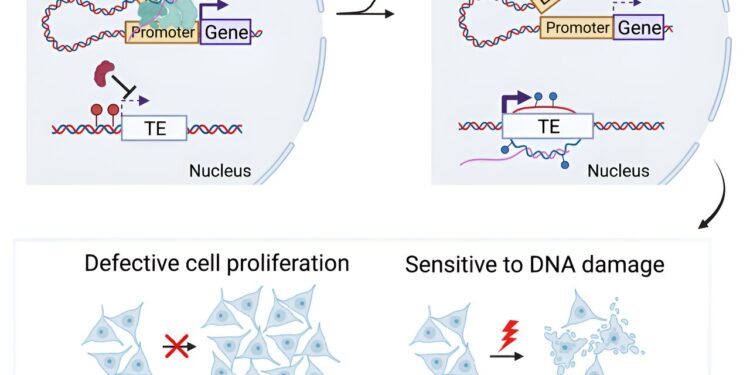
Yao and his colleagues wanted to examine what happens when TDP-43 clumps together in the cytoplasm and therefore doesn’t do what it’s supposed to do in the nucleus. They therefore created cell lines whose TDP-43 gene is chronically refused. Cells divide more slowly and are more susceptible to DNA damage, but they remain alive.
A detailed analysis of what’s going wrong shows how critical TDP-43 is and how it might deserve the name “guardian of the genome” just as much as the tumor suppressor p53.
Now let’s move on to the zombies. In this case, zombies are transposable elements, sometimes mobile retrovirus-like sequences that make up a large part of our genomes.
Transposable elements (TEs) are usually inactive and not expressed, but if they are activated, several problems can happen to cells, such as DNA damage, inflammation, and senescence. Cells therefore have means to prevent ETs from taking over.
The authors’ analysis shows that in the absence of TDP-43, a large number of TEs are activated. While other labs have shown that troubling things happen to TEs without TDP-43, the elements themselves are difficult to analyze because their sequences are so similar to each other. By examining the genomic context, this article was able to assess which TE families are activated.
Yao and colleagues are also analyzing the genome-wide effects of TDP-43 depletion. TDP-43 normally maintains R-loops (three-stranded RNA-DNA structures in the nucleus) and facilitates their processing. Without TDP-43, the R-loop landscape changes. Some genes show more and others less.
Patterns of DNA hydroxymethylation – an epigenetic modification associated with active genes – also change. Additionally, the absence of TDP-43 appears to interfere with long-range interactions between enhancers and promoters, which are essential for correct gene expression.
“Our results suggest that besides TDP-43, other RNA-binding proteins could play this type of genome-wide role,” says Yao.
As for next steps, Yao’s team plans to analyze post-mortem tissue samples from Alzheimer’s and ALS patients, as it is important to confirm that the same mechanism works in human diseases.
From a therapeutic perspective, some inconclusive clinical trials have tested antiretrovirals in ALS, but Yao says it might be better to go after specific R-loops. He is developing a strategy combining CRISPR/dCas with an enzyme targeting RNA/DNA hybrid structures (RNAse H).
More information:
Yingzi Hou et al, chronic TDP-43 deficiency leads to dysregulation of transposable elements and gene expression by affecting crosstalk of the R-loop and 5hmC, Cell Reports (2024). DOI: 10.1016/j.celrep.2023.113662
Provided by Emory University
Quote: The TDP-43 protein keeps genetic zombies at bay: new insights into the mechanisms of neurodegenerative diseases (January 24, 2024) retrieved January 24, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.