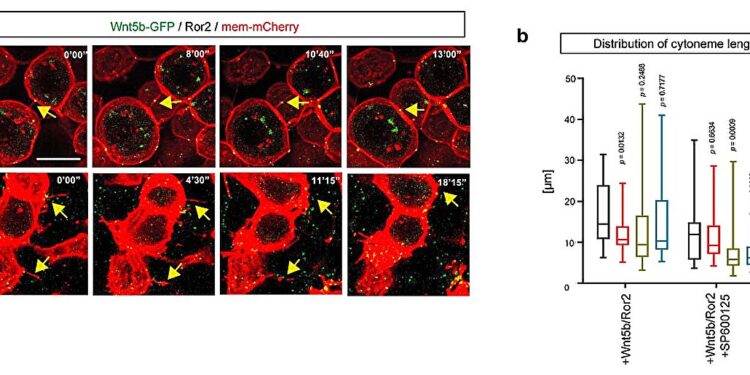
Cytoneme modulators for Wnt5b/Ror2 transport. A. Time series of cytonemes in wild-type zebrafish embryos. Embryos were injected with Wnt5b-GFP/Ror2/mem-mCherry into the clone. At 6hpf, embryos were treated with DMSO or JNK inhibitor SP600125 from 0’00” to 35’00” and imaged live. Yellow arrows indicate cytoneme retraction at the corresponding times. b. Quantitative analysis of cytoneme length at different time points in zebrafish embryos was processed as shown in (a). Credit: Nature (2023). DOI: 10.1038/s41586-023-06850-7
Researchers at the University of Exeter, UK, have discovered complex mechanisms of ligand-receptor complex transport via specialized protrusions carrying signaling components between cells, challenging the conventional understanding of cellular responsiveness based solely on receptor expression.
In their paper titled “Cytoneme-mediated transport of active Wnt5b–Ror2 complexes in zebrafish,” published in Naturethe team investigated the mechanisms behind cellular communication in the embryo, focusing specifically on the role of chemical signaling via the Wnt-planar cell polarity pathway in zebrafish.
A research paper published in the same journal issue summarizes the words of Chengting Zhang and his colleagues.
Cellular communication during embryonic development occurs primarily through chemical signaling, where ligands released by signal-producing cells interact with receptors on target cells. In the zebrafish embryo, Wnt5b binds to the Ror2 receptor to trigger the Wnt–planar cell polarity (PCP) signaling pathway to regulate tissue polarity and cell migration.
Using fluorescent labeling, the researchers found evidence that Wnt5b and Ror2 form active complexes in producer cells and are transferred via cytonemes to neighboring cells. Cytonemes are tubular or tubulovesicular cellular filopodia structures involved in several cellular functions. They act as a kind of probe or transport corridor, extending beyond the edge of the cell to interact with other cells.
Cytoneme-transported complexes retained their functionality, activating Wnt–PCP signaling in recipient cells, even though they lack functional Ror2 receptors. The results challenge the conventional view of tissue responsiveness based solely on receptor expression, introducing the transfer of signaling complexes between cells through the cytoneme as a recently discovered method of intercellular communication.
The experiments involved zebrafish lacking Ror1 or Ror2 and double mutants to understand the roles of these receptors. Wnt5b-Ror2 protein signaling in transmitter cells influenced cytoneme formation, resulting in fewer but longer tubulovesicular structures.
The study demonstrated that Wnt5b-Ror2 complexes transported via cytonemes maintained their activity in target cells, activating the intercellular signaling activity of the recipient cell. Cytoneme-mediated dissemination of signaling proteins influenced gene convergence, extension, and expression during early stages of zebrafish development.
The discovery of a novel mechanism of cell-to-cell communication in embryogenesis may have considerable clinical significance in human embryogenesis, prenatal development and pathological conditions, congenital disabilities, and intercellular communication in cancer cells.
Further research is needed to replicate and verify such an extraordinary discovery. Beyond replication of results, the study involves other mechanisms outside the experimental setting of the study, such as a molecular signaling method for uptake of a protein delivered by the cytoneme or catalyst to generate longer cytonemes. If confirmed, it will also require enormous research into the potential implications of this previously unknown cellular signal sharing capability on previous research.
More information:
Chengting Zhang et al, Cytoneme-mediated transport of active Wnt5b–Ror2 complexes in zebrafish, Nature (2023). DOI: 10.1038/s41586-023-06850-7
Embryonic zebrafish cells do not need receptors to receive signals from a distance, Nature (2023). DOI: 10.1038/d41586-023-03659-2
© 2023 Science X Network
Quote: New intercellular signal sharing pathway discovered in zebrafish experiments (December 27, 2023) retrieved December 27, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.