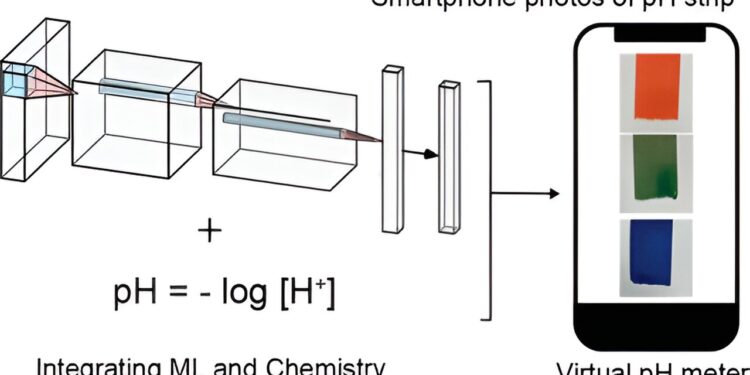
Graphical summary. Credit: Journal of Chemical Education (2023). DOI: 10.1021/acs.jchemed.3c00589
Researchers at North Carolina State University have developed a week-long high school curriculum that helps students quickly understand the concepts of color chemistry and artificial intelligence, while igniting their curiosity about science and the world around them.
To test whether a short high school science module could actually teach students something about chemistry (a notoriously tricky subject) and artificial intelligence (AI), the researchers designed a relatively simple experiment involving pH levels, which reflect the acidity or alkalinity of a liquid. solution.
When testing pH levels on a test strip, the color conversion charts provide a handy reference: more acidic solutions make the test strips red when high acidity is present and make them yellow and green when the levels are high. acid decreases. The test strips turn dark purple when liquids are very alkaline and turn blue and dark green when alkaline levels decrease.
Numerical ranges for pH range from 0 to 14, with seven being neutral (about the level of your home’s tap water) and lower amounts reflecting greater acidity, with higher numbers reflecting greater alkalinity.
“We wanted to answer the question: ‘Can we use machine learning to read pH strips more accurately than visually?'” said Yang Zhang, assistant professor of textile engineering, chemistry and science and co-corresponding author of a paper describing the work. “It turns out that the AI predictive model trained by the students was about 5.5 times more accurate than the visual interpretations.”
Students used their cell phone cameras to take photos of pH test strips after wetting them in various everyday liquids (drinks, pond or lake water, cosmetics, etc.) and visually predicts their pH values. They also received test strips from instructors with known pH levels taken with sophisticated instruments and predicted them visually.
“We wanted students to think about the real-world implications of this type of testing, for example in underdeveloped places where clean water might be a problem,” Zhang said. “You may not have a fancy instrument, but you really want to know if the pH level is below 5 versus 7.”
The students entered their data into free machine learning software called Orange, which has no lines of code, making it easier for novices to work. They worked to convert test strip images and pH values into predictions, with machine learning improving accuracy as it learned to delineate the most subtle changes in test strip color with pH values. corresponding pH.
The students then compared their machine learning pH level predictions with their visual predictions and found that the AI predictions, while imperfect, were much closer to the actual pH value than their predictions. visual.
The researchers also surveyed the students before and after the week-long program and found that they reported being more motivated to learn and better informed about chemistry and AI.
“Students were able to see first-hand the relevance of cutting-edge technology when applied to real-world problems and scientific advancements,” said Shiyan Jiang, assistant professor of learning design and technology at NC State and co -corresponding author of the article.
“This hands-on application not only enhances their understanding of complex scientific concepts, but also inspires them to explore innovative solutions, fostering a deeper appreciation of the intersection of technology and cutting-edge science, particularly chemistry. “
“On the chemistry side, there are many similar color chemistry concepts that we can teach this way,” Zhang said. “We can also expand this program to include more students.”
The research is published in the Journal of Chemical Education.
More information:
Shiyan Jiang et al, Integrating machine learning and color chemistry: developing a high school curriculum toward solving real-world problems, Journal of Chemical Education (2023). DOI: 10.1021/acs.jchemed.3c00589
Provided by North Carolina State University
Quote: New high school program teaches color chemistry and AI simultaneously (December 7, 2023) retrieved December 7, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.