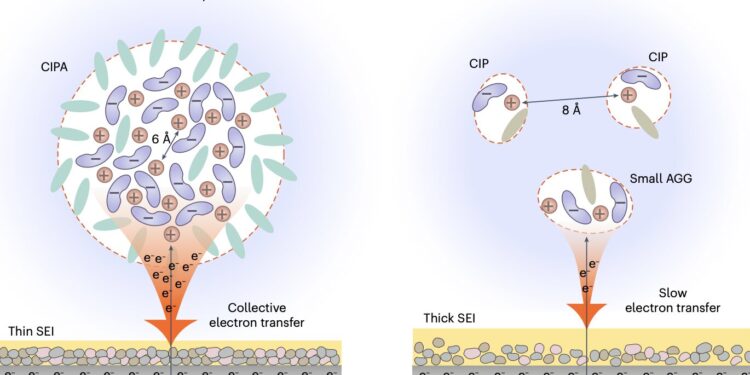
Schematics of solvation structures and interfacial reaction mechanisms of CIPA electrolyte and conventional LHCEs. Credit: Jie et al.
Lithium-metal batteries could have significantly higher energy densities than lithium-ion batteries, which are the dominant battery technology on the market today. However, lithium-metal cells generally have significant limitations, the most notable being their short cycle life.
Researchers from the University of Science and Technology of China and other institutes recently presented a new electrolyte design that could be used to develop high-performance lithium-metal cells with longer cycle life. This electrolyte, presented in a paper published in Natural energyexhibits a unique nanoscale solvation structure, with ion pairs densely packed into close-packed ion pair aggregates (CIPAs).
“The main goals of our recent work are to significantly accelerate the practical applications of lithium-metal batteries and provide a deep mechanistic understanding of this complex system,” Professor Shuhong Jiao, co-author of the paper, told Tech Xplore.
“Lithium-metal batteries are the holy grail of the battery industry and are considered a promising next-generation technology because they have an ultra-high energy density, theoretically above 500 Wh/kg. This is more than 2 times more than the current lithium-ion batteries that dominate the battery market, which means that if we can replace lithium-ion batteries with lithium-metal batteries, the range of electric vehicles can be doubled per charge.”
Lithium-metal batteries marketed so far have a very limited cycle life of about 50 cycles, which is significantly lower than that of commercial lithium-ion batteries, which can generally maintain their good performance for about 1,000 cycles. The reasons for this shorter cycle life are the growth of lithium dendrites, the high reactivity of lithium metal, and the high-voltage transition metal cathodes, which collectively cause the electrolyte to constantly degrade.
“Despite the great efforts of researchers worldwide, the performance of lithium-metal batteries is still far from satisfactory (>500 Wh/kg, 1,000 cycles),” said Prof. Jiao. “The main cause is that the interfaces between the electrolyte and the electrodes (i.e., the anode-electrolyte interface and the cathode-electrolyte interface) cannot be completely stabilized as in the case of lithium-ion batteries. Constant and severe degradation of the electrolyte always occurs during battery operation.”
About five years ago, Professor Jiao and his colleagues designed an electrolyte that could simultaneously stabilize the anode-electrolyte and cathode-electrolyte interfaces in lithium-metal battery cells, thereby suppressing electrolyte degradation. Their electrolyte design was based on early research on microscopic physicochemical processes inside lithium-metal batteries.
“An electrolyte is a key component of lithium-metal batteries because it can adjust the chemistry/structure of the SEI and thus guide the plating behavior of the lithium-metal, ultimately dictating the battery performance,” explained Prof. Jiao.
“For the sake of practical application, we tried to achieve this using cheap components. The countless works of other researchers in this field also inspired us a lot, as they introduced many new classes of electrolytes like highly concentrated electrolyte, high concentration localized electrolyte, low solvation electrolyte and liquefied gas electrolyte, etc.”
To carry out this recent study, Professor Jiao and her research group teamed up with other teams capable of performing theoretical calculations and characterizing electrolytes at the microscopic scale. Their collaborative efforts ultimately led to the design of a new class of electrolytes capable of extending the life of lithium-metal batteries.
The electrolytes they designed are made of commercially available and affordable molecules. Their main characteristic is their unique solvation structure.
“The solvation structure is a crucial inherent characteristic of an electrolyte, as it governs the interfacial behavior of the electrolyte, such as its interfacial reaction mechanism that controls the formation of SEI and thus the chemistry and structure of SEI,” said Prof. Jiao.
“The solvation structure of the electrolyte has been so far intensively tuned at the microscopic level in the peer-reviewed scientific literature, especially the first solvation shell of the lithium ion, but the structural tuning beyond this scale, namely the second solvation shell and beyond, is largely neglected.”
The recent study by Professor Jiao and colleagues has paved the way for tuning the solvation structure of an electrolyte at the mesoscopic level. Their unique design focuses specifically on the interaction between ion pairs underlying the formation of the electrolyte’s aggregate structure.
“Our electrolyte is composed of large, compact aggregates, formed by the dense packing of lithium-anion ion pairs with coordinate bonding between them, which we define as the compact ion-pair aggregate (CIPA),” said Prof. Jiao. “This is in sharp contrast to the dominance of small aggregates and separated ion pairs in the high-concentration localized electrolyte, a class of advanced electrolytes with the best battery performance to date, opening a new avenue for electrolyte design.”
The new electrolyte designed by this research team notably exhibits a unique collective reduction on the lithium-metal anode. This means that the anion clouds of the CIPA structure are rapidly reduced (i.e. decomposed) on the lithium surface, forming inorganic compounds such as Li2O and LiF, as well as a thin, stable SEI, which in turn suppresses the constant decomposition of the electrolyte.
“Due to the unique behavior of collective electron transfer, our electrolyte forms a thin and conformal SEI with low organic content and rich in inorganic components with uniform distribution, which can promote the homogeneous flow of lithium ions inside the SEI and the dendrite-free lithium deposition,” said Professor Jiao. “This leads to homogeneous and compact lithium deposition, which decreases the specific areas of the lithium-metal anode to further suppress the electrolyte decomposition.”
In addition, the researchers’ newly designed electrolyte simultaneously exhibits good oxidation stability and suppresses the dissolution of transition metal elements in the cathode, thereby improving the stability of the cathode interface. The stabilization of this interface, as well as that of the electrolyte-lithium interface, was found to result in stable cycling over a prolonged number of cycles.
“The mesoscopic solvation structure introduced in our paper leads to a new class of electrolytes, opening a new avenue for the design of lithium-metal battery electrolytes,” said Professor Jiao.
To assess the potential of their new electrolyte, the researchers used it to create a 500 Wh/kg lithium-metal cell. In initial tests, the cell was found to retain 91% of its energy after 130 operating cycles. In the future, this new electrolyte design could be replicated and tested by other researchers around the world to further assess its potential to extend the life of lithium-metal batteries.
“We are now considering to further extend the cycle life of lithium-metal cells from 500 Wh/kg to over 1,000 cycles,” added Professor Jiao. “On the other hand, we are still exploring the new battery system to achieve much higher energy density with long cycle life, such as ≥600 Wh/kg with 100-200 cycles. All these basic scientific research studies are valuable to realize the deployment of lithium-metal batteries in many fields.”
More information:
Yulin Jie et al., Towards long-life lithium metal cells of 500 Wh kg−1 via compact ion-pair cluster electrolytes, Natural energy (2024). DOI: 10.1038/s41560-024-01565-z
© 2024 Science X Network
Quote:New electrolyte design promises longer-lasting lithium-metal batteries (2024, August 18) retrieved August 19, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.