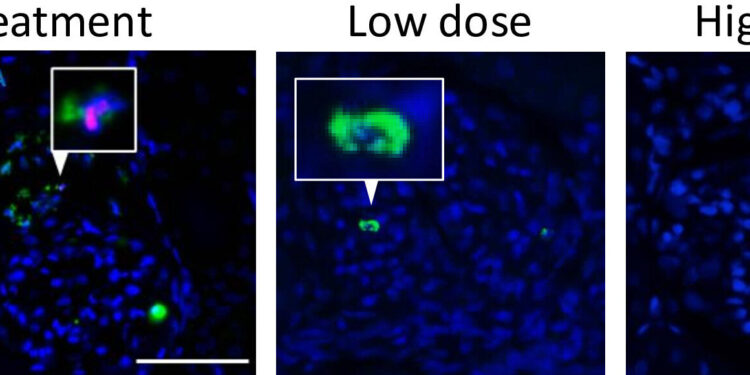
Ameliorative effects of MOD06051. In the glomeruli of the disease group, neutrophils (green) positive for the NETs marker citrullinated histone H3 (Cit-H3; red) are observed. Cit-H3 was not observed in the infiltrated neutrophils in the low-dose and high-dose treatment groups. (Yuka Nishibata, et al. Nature Communications. August 22, 2024). Credit: (Yuka Nishibata, et al. Nature Communications. August 22, 2024)
A newly developed compound that reduces harmful inflammation caused by overactive neutrophils in rats shows great potential as a safer treatment for various inflammatory diseases in humans.
Neutrophils are the most abundant white blood cells in the human body and play a crucial role in the immune response. These immune cells help fight infections by engulfing pathogens and releasing enzymes that kill the invaders.
But while they are essential for fighting infections, neutrophils can also become overactive, leading to a variety of inflammatory diseases. When activated by an infection, neutrophils can release neutrophil extracellular traps (NETs), web-like structures made of DNA and proteins, which trap and kill pathogens as part of the host’s normal defense mechanism. However, excessive NET formation can cause significant tissue damage, contributing to inflammation.
A team of researchers from Hokkaido University and Alivexis, Inc. studied a newly developed drug candidate, MOD06051, which reduces harmful inflammation in rat models by targeting neutrophils. The results of their joint research are published in Nature Communications.
“We discovered that MOD06051 functions as a selective inhibitor of cathepsin C (CatC), a key regulator that activates several enzymes within neutrophils known as neutrophil serine proteases (NSPs),” said co-author Yoh Terada of Alivexis, Inc. “One of these NSPs is neutrophil elastase, an enzyme involved in pathogen destruction but also a critical factor for NET formation.”
Blocking the CatC enzyme can reduce the harmful activity of neutrophils and alleviate diseases caused by hyperactive neutrophils. (Yuka Nishibata, et al. Nature Communications. August 22, 2024). Credit: (Yuka Nishibata, et al. Nature Communications. August 22, 2024)
Scientists have found that inhibiting CatC reduces the active form of neutrophil elastase and decreases the ability of neutrophils to form NETs. Excessive NET formation has been associated with several diseases, including vasculitis, lupus, rheumatoid arthritis, and diabetes.
“When we tested the compound in rats with a specific type of vasculitis, it reduced the severity of the disease, which was reflected by reduced inflammation and damage to blood vessels, particularly in the kidneys and lungs,” said Professor Akihiro Ishizu, who led the study.
“Our results suggest that CatC inhibition holds promise as a novel treatment strategy to reduce neutrophil overactivation and improve conditions in diseases where hyperactive neutrophils and excessive NET formation play a critical role. This approach differs from current treatments that may have broader immunosuppressive effects.”
Current treatments for inflammatory diseases often involve the use of glucocorticoids and immunosuppressive drugs that suppress the activity of the immune system as a whole and can lead to secondary immunodeficiency, increasing the risk of opportunistic infections. By specifically targeting the activation of multiple NSPs through CatC inhibition without broadly suppressing the immune system, MOD06051 potentially offers a safer alternative that could reduce the risk of infections and other side effects.
These results pave the way for further research and clinical trials to evaluate the safety and efficacy of MOD06051 in humans. The team is optimistic that this new approach promises to provide safer and more effective therapies to patients worldwide suffering from a variety of inflammatory diseases, thereby improving their quality of life.
More information:
Cathepsin C inhibition reduces neutrophil serine protease activity and ameliorates activated neutrophil-mediated disorders, Nature Communications (2024). DOI: 10.1038/s41467-024-50747-6
Provided by Hokkaido University
Quote:New compound shows great potential for patients with neutrophil-associated inflammation (2024, August 22) retrieved August 22, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.