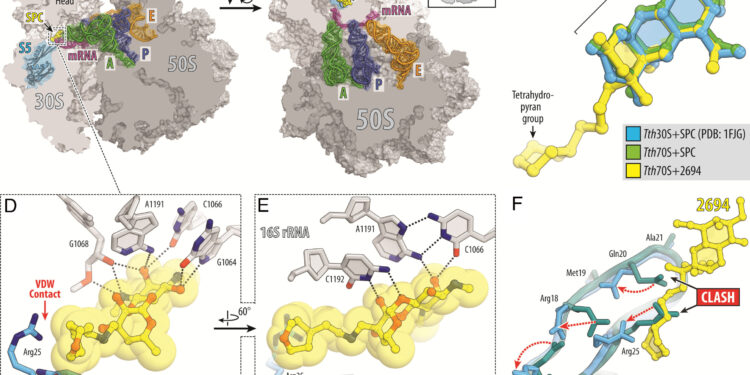
Structure of SPC and eAmSPC 2694 (2694) in complex with 70S ribosome, mRNA and tRNA. (A And B) Overview of the SPC/2694 binding site (yellow) in the T. thermophilus Ribosome 70S seen as a cross section from two different perspectives. The 30S subunit is shown in light gray, the 50S subunit is in dark gray, the mRNA is in magenta, and the A, P, and E site tRNAs are colored in green, dark blue, and orange, respectively. The small ribosomal protein S5 is highlighted in blue. The view in (A) is a cross section of the 70S ribosome. The view in (B) come from High after removing the head of the 30S subunit and the protrusions of the 50S subunit, as indicated by the Insert. (VS) Superposition of the SPC bound to the ribosome (green) and 2694 (yellow) in the presence of tRNA with the previous structure of the SPC bound to the 30S ribosomal subunit of T. thermophilus (blue, PDB entry 1FJG in ref. 21). The structures were aligned based on helix 34 of the 16S rRNA. (D And E) Close-up views of the 2694 interactions with helix 34 of the decoding center on the 30S ribosomal subunit. THE E.coli nucleotide numbering in 16S rRNA is used. Potential H-bond interactions are indicated by dashed lines. Note that the extended tetrahydropyranyl fragment of 2694 establishes van der Waals interactions with residue Arg25 of ribosomal protein S5. (F) Superposition of structures of ribosomal protein S5 in the absence (teal) and presence of SPC or 2694 bound to the ribosome (blue). Although binding of SPC/2694 to the bacterial ribosome does not cause significant rearrangements of the S5 protein loop, the side chains of several residues in the loop (Arg18, Gln20, Arg25, Arg27) are displaced (red dotted arrows) to avoid steric residues. obstacle with drugs linked to ribosomes. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2314101120
Scientists at St. Jude Children’s Research Hospital are tackling antibiotic resistance in Mycobacterium abscessus (Mab). This naturally antibiotic-resistant pathogen is becoming increasingly prevalent, highlighting the urgent need for new treatments. To address this problem, scientists have designed new versions of the drug spectinomycin that overcome efflux, the main mechanism causing resistance. The work is published in Proceedings of the National Academy of Sciences.
Mab infections are increasingly common in health care settings. Such infections can be dangerous for patients who have compromised lung function, as in cystic fibrosis, or who are immunologically compromised, as in childhood cancer. These infections are treated with long courses of antibiotics and can lead to poor outcomes.
The emergence of Mab and other similar pathogens presents a growing and deeply concerning threat to public health, as there are few effective therapeutic options and a limited drug development pipeline.
“We chemists are in a race against pathogens. We are making more powerful antibiotics and the pathogens are becoming more resistant,” said corresponding author Richard Lee, Ph.D., Department of Chemical Biology and Therapeutics of St. Jude.
St. Jude scientists modified the natural antibiotic spectinomycin to create comparable but structurally distinct analogues of N-ethylene-linked aminomethylated spectinomycins (eAmSPC). These synthetically created eAmSPCs are up to 64 times more potent against Mab than standard spectinomycin.
“By rearranging the molecule through structure-based drug design, we and our collaborators adapted the antibiotic to increase its activity,” Lee added.
Overcoming efflux to make a more effective antibiotic
Thanks to their work, the scientists discovered the mechanism of action by which eAmSPCs are more effective: they bypass efflux. Efflux is the process that cells use to get rid of a drug (imagine pumping water from a flooded basement) and is an important mechanism by which cells become resistant to therapy.
The N-ethylene bond structure of eAmSPCs plays a critical role in how compounds avoid efflux, suggesting that longer bonds change how the compound is pumped out of the cell. This ultimately shifts the balance towards higher concentrations of eAmSPC in the cell and thus improves antimicrobial efficacy.
“Over the past two decades, we have seen a massive increase in the number of infections caused by nontuberculous mycobacteria like Mab,” said co-first author Gregory Phelps, PharmD, St. Jude Graduate School of Biomedical Sciences. “We had a starting point with this natural antibiotic that, through modifications, we made much more effective against this clinically relevant pathogen.”
The researchers also found that eAmSPCs work well with various classes of antibiotics used to treat Mab and retain activity against other mycobacterial strains. This work demonstrates that eAmSPCs should be further studied and developed because once tolerability and safety issues are resolved, these compounds could become next-generation therapeutics.
“Incentivizing pharmaceutical companies to develop new antibiotics is difficult for several economic reasons,” Phelps said. “If we can strengthen the drug pipeline against this difficult-to-treat bacteria, we can potentially make a difference for patients like those we have here at St. Jude, who increasingly face limited or even limited treatment options. non-existent.”
More information:
Gregory A. Phelps et al, Development of 2nd generation aminomethyl spectinomycins that overcome native efflux in Mycobacterium abscessus, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2314101120
Provided by St. Jude Children’s Research Hospital
Quote: A new approach can combat antibiotic resistance in Mycobacterium abscessus (January 5, 2024) retrieved January 5, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.