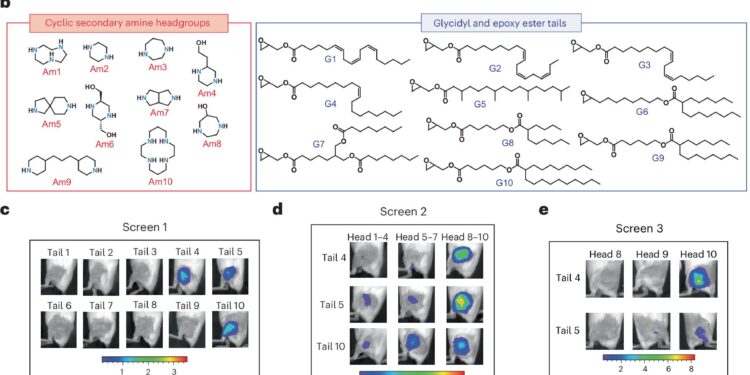
Synthesis and screening of the AMG library. Credit: Nature Nanotechnology (2025). DOI: 10.1038/s41565-025-02044-6
A new delivery particle developed at MIT could make mRNA vaccines more effective and potentially reduce the cost per vaccine dose.
In mouse studies, the researchers showed that an mRNA flu vaccine delivered with their new lipid nanoparticle could generate the same immune response as mRNA delivered by nanoparticles made with FDA-approved materials, but at about 1/100 the dose.
“One of the challenges with mRNA vaccines is their cost,” says Daniel Anderson, professor in the MIT Department of Chemical Engineering and member of the Koch Institute for Integrative Cancer Research and the Institute of Medical Engineering and Science (IMES).
“When you think about the cost of making a vaccine that could be widely distributed, it can really add up. Our goal has been to try to make nanoparticles that can give you a safe and effective vaccine response, but at a much lower dose.”
If researchers used their particles to deliver a flu vaccine, they could also be used for vaccines against COVID-19 and other infectious diseases, they say.
Anderson is the lead author of the study, which appears in Nature Nanotechnology. The paper’s lead authors are Arnab Rudra, visiting scientist at the Koch Institute; Akash Gupta, researcher at the Koch Institute; and MIT graduate student Kaelan Reed.
Efficient delivery
To prevent mRNA vaccines from breaking down in the body after injection, they are packaged in a lipid nanoparticle, or LNP. These fatty spheres help the mRNA enter cells so that it can be translated into a protein fragment from a pathogen such as influenza or SARS-CoV-2.
In the new study, the MIT team sought to develop particles that could induce an effective immune response, but at a lower dose than particles currently used to deliver mRNA COVID-19 vaccines. This could not only reduce costs per vaccine dose, but also help mitigate potential side effects, researchers say.
LNPs generally consist of five components: an ionizable lipid, cholesterol, an auxiliary phospholipid, a polyethylene glycol lipid, and mRNA. In this study, researchers focused on ionizable lipid, which plays a key role in vaccine strength.
Based on their knowledge of chemical structures that could improve delivery efficiency, the researchers designed a library of novel ionizable lipids. These contained cyclic structures, which may help improve mRNA delivery, as well as chemical groups called esters, which the researchers believe could also help improve biodegradability.
The researchers then created and examined numerous combinations of these particle structures in mice to see which could most efficiently transmit the gene for luciferase, a bioluminescent protein. Then they took their best-performing particle and created a library of new variants, which they tested in another selection round.
From these screens, the main LNP that emerged was what the researchers called AMG1541. One of the key features of these new LNPs is that they are more effective in dealing with a major barrier to particle delivery, known as endosomal escape. Once LNPs enter cells, they are isolated into cellular compartments called endosomes, from which they must exit to deliver their mRNA. The new particles achieve this more efficiently than existing LNPs.
Another advantage of the new LNPs is that the ester groups present in the tails make the particles degradable once they have delivered their cargo. This means they can be cleared from the body quickly, which researchers believe could reduce the side effects of the vaccine.
More powerful vaccines
To demonstrate the potential applications of the AMG1541 LNP, researchers used it to deliver an mRNA influenza vaccine in mice. They compared the effectiveness of this vaccine to a flu vaccine made with a lipid called SM-102, approved by the FDA and used by Moderna in its COVID-19 vaccine.
Mice vaccinated with the new particles generated the same antibody response as mice vaccinated with the SM-102 particle, but only 1/100 of the dose was needed to generate this response, the researchers found.
“It’s almost a hundred times lower dose, but you generate the same amount of antibodies, which can significantly reduce the dose. If this translates to humans, it should also significantly reduce the cost,” says Rudra.
Further experiments revealed that the new LNPs are better able to deliver their cargo to a critical type of immune cell called antigen-presenting cells. These cells chop up foreign antigens and display them on their surface, which signals other immune cells such as B and T cells to activate against that antigen.
New LNPs are also more likely to accumulate in lymph nodes, where they encounter many more immune cells.
Using these particles to deliver mRNA flu vaccines could allow vaccine developers to better match the flu strains that circulate each winter, researchers say.
“With traditional flu vaccines, they have to start being made almost a year in advance,” Reed says. “With mRNA, you can start producing it much later in the season and get a clearer idea of what the circulating strains will be, and that can help improve the effectiveness of flu vaccines.”
The particles could also be suitable for vaccines against COVID-19, HIV or any other infectious disease, the researchers say.
“We found that they work much better than anything reported so far. That’s why, for any intramuscular vaccine, we believe our LNP platforms could be used to develop vaccines against a number of diseases,” says Gupta.
More information:
Arnab Rudra et al, Degradable cyclic amino alcohol ionizable lipids as vectors for potent mRNA influenza vaccines, Nature Nanotechnology (2025). DOI: 10.1038/s41565-025-02044-6.
Provided by the Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news in MIT research, innovation and education.
Quote: Nanoparticles that improve mRNA delivery could reduce vaccine dosage and costs (November 8, 2025) retrieved November 8, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.