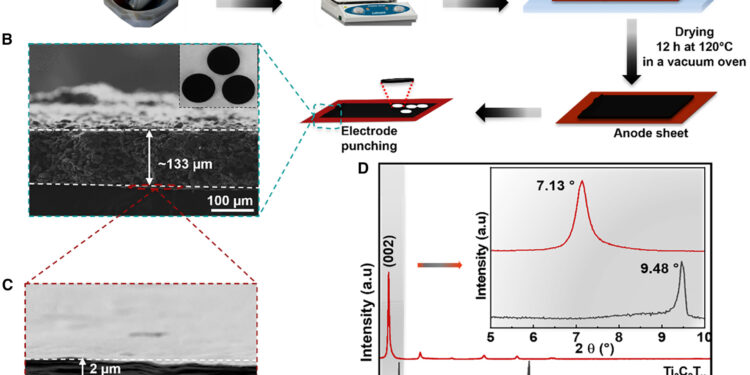
Schematic view of the different steps followed for the preparation of the electrode, the assembly of the button cell and the characterization of the Ti3C2Tx MXene film. Credit: Cell Reports Physical Sciences (2025). DOI: 10.1016/j.xcrp.2025.102874
The vast majority of consumer electronics devices use lithium-ion batteries, and with each generation, these devices are designed smaller, lighter and with longer lifespans to meet growing consumer demands. Each new iteration also brings the batteries that power the devices closer to the limits of their size, weight and performance.
Researchers are constantly testing new approaches and new materials to manufacture lightweight, high-performance components. The final contender is MXene, a type of two-dimensional metallic conductive nanomaterial discovered by Drexel University researchers that recently demonstrated its potential as a current collector, the part of the battery that directs electrical current to its electrodes.
A recent paper from Drexel researchers reports that a current collector made of MXene film could reduce the weight and thickness of the battery while improving its available capacity.
Published in the journal Cellular Reports Physical Sciencesthe paper reports that MXene current collectors perform as well as the copper foils used in current lithium-ion batteries, but are three to four times thinner and about 10 times lighter.
Using them to make battery components would reduce the overall weight contribution of inactive materials, allowing more energy-storing materials to be used without increasing battery weight, thereby improving battery capacity.
Researchers also demonstrated that MXene current collectors can be easily recycled for use in other batteries, an important step toward reducing battery waste and conserving limited material resources.
Current collectors are critical to battery performance because they direct the flow of electrons inside the battery, directing them to and from the electrode, which translates chemical energy into electrical current that powers electronic devices. They also contribute a large part to the weight of a battery, representing almost 15% of its total weight.
“Recent advances in battery technology are focused on improving capacity while reducing weight,” said Yury Gogotsi, Ph.D., Distinguished University and Bach Professor in Drexel’s College of Engineering, who led the research.
“But the field has also widely recognized the importance of finding recyclable alternatives to current battery components to ensure their sustainable manufacturing. Our results suggest that MXene materials could be good candidates for use in batteries of the future.”
MXenes have been tested in dozens of applications, including several in energy storage, since their discovery at Drexel more than a decade ago. Their suitability for use as a current collector is linked to their exceptional electrical conductivity, excellent flexibility and high mechanical strength. Mxenes also remain electrochemically stable in acidic and corrosive electrolytes and are dispersible in water, allowing easy processing.
“This is an exciting finding because MXenes are compatible with a variety of electrode materials, so they have the potential to improve next-generation batteries without requiring significant structural modifications,” said Professor Patrice Simon, Ph.D., co-author of the research from the University of Toulouse in France.
Final component testing examined the cyclic stability and recyclability of the MXene current collector. After eight weeks of continuous charging and discharging, the MXene-graphite electrode retained good adhesion; the graphite active material remained uniformly distributed and did not detach from the MXene film.
The MXene current collector also retained its layered structure, showing no degradation. Using a simple, environmentally friendly recycling process developed by the team, the electrode was taken apart and put back together using salvaged materials for the current collector. Electrochemical tests confirmed that its performance remained unchanged.
“As battery materials become increasingly scarce and sustainability and the circular economy become increasingly important, it will be critical to design components that can be reused,” said Yuan Zhang, Ph.D., a postdoctoral researcher in Gogotsi’s lab and co-author of the research.
“Thanks to their exceptional electrochemical durability, MXenes can be recycled without losing much of their exceptional properties.”
The investigation was led by Sokhna Dieng, a Schlumberger Future Fellow in Gogotsi’s lab, who contributed to the work as part of her doctoral research. She plans to continue exploring MXenes as conductive additives and other passive components in batteries that can improve performance and also enhance safety by preventing dendrite growth.
“We envision batteries with MXene components one day being used in wearable and wearable microelectronics, where size and weight are absolutely critical and the amount of material required is minimal,” Gogotsi said.
“Another potential use is in systems where low weight is essential, such as drones or other flying vehicles.”
More information:
Sokhna Dieng et al, MXene Current Collectors for Recyclable Batteries with Enhanced Capacity, Cellular Reports Physical Sciences (2025). DOI: 10.1016/j.xcrp.2025.102874
Provided by Drexel University
Quote: MXene current collectors could reduce size and improve recyclability of Li-ion batteries (October 13, 2025) retrieved October 13, 2025 from
This document is subject to copyright. Except for fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.