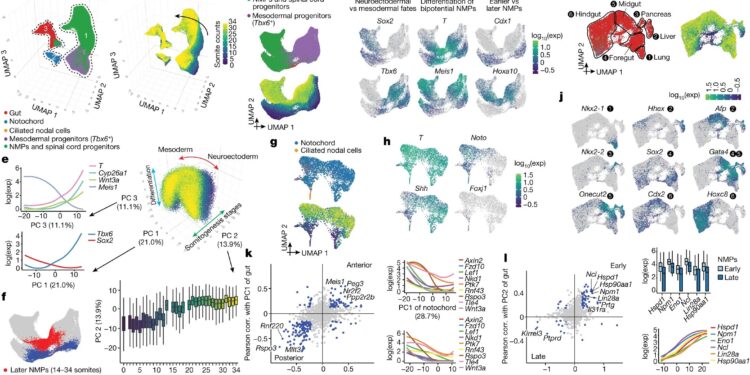
Transcriptional heterogeneity in the posterior embryo at the onset of somitogenesis. A, Reintegrated 3D UMAP of 121,118 cells from selected later embryonic cell types at the start of somitogenesis (somite numbers 0 to 34; E8 to E10). Three clusters are identified. bThe same UMAP as in Acolored by somite accounts. vsUMAP 2D reintegrated cluster 1 cells. dThe same UMAP as in vs, colored by marker gene expression for MPN subpopulations (Supplementary Table 12). Exp, expression. e3D visualization of the three principal components of gene expression variation in group 1. Correlations between the three principal components and normalized expression of selected genes (left) or somite number (bottom). FThe same UMAP as in vswith earlier (not = 4,949 cells) and later (not= 3,910 cells) NMP highlighted. NPM: T+(raw number ≥ 5) and Meis1− (raw number = 0). gUMAP 2D reintegrated cluster 2 cells. hThe same UMAP as in gcolored by the expression of a marker gene for the notochord or ciliated nodal cells (Foxj1+). IUMAP 2D reintegrated cells from group 3. Black circles highlight intestinal cell subpopulations. jThe same UMAP as in Icolored by marker gene expression for intestinal cell subpopulations (Supplementary Table 12). k, Left, Pearson correlation (corr.) with PC1 from notochord or gut for highly variable genes. Right, gene expression of selected Wnt signaling genes relative to PC1 from the notochord or intestine. IOn the left, fold changes between early and late MPNs and Pearson correlation with gut PC2 are plotted for highly variable genes. On the right, gene expression of selected genes (several MYC targets, Lin28a And Hsp90aa1) versus early and late MPNs or intestinal PC2. In vs,g,I, cells are colored either by the initial annotations or by the number of somites. Boxplots in e (not = 98,545 cells) and I (not= 8,859 cells) represent the interquartile range (IQR) (25th, 50th, and 75th percentile) and the whiskers represent 1.5 × IQR. Credit: Nature(2024). DOI: 10.1038/s41586-024-07069-w
During its gestation, the house mouse begins as a single fertilized cell, and three weeks later it is ready to enter the world as a free-living puppy made up of more than 500 million cells.
Scientists are eager to understand how the genome of the house mouse, Mus musculus, orchestrates this routine but astonishing transformation. Although their pregnancies are much shorter, mice share many evolutionary commonalities with humans. It not only offers a useful scientific model of mammalian prenatal development, but could also reveal some basic insights into what happens during human pregnancies.
However, it has been difficult to determine the genetic activities that determine the timing and appearance of the hundreds of cell types that make up a complete newborn mouse, with all its parts in the right place. Partly because of this large number of cells, the genetic drivers of early mouse embryonic development have already been roughly sampled at fairly long intervals, such as during daily observations.
New techniques allow scientists to better profile what is happening genetically in nuclei – control centers inside individual cells – at shorter, precise intervals and for a total of more than 12 million cells in 83 embryos of mice. This allowed them to obtain, at the single-cell level, an improved and annotated time-lapse of mouse prenatal development.
Their analysis began when each embryo first formed into a multilayered structure, the gastrula, and continued until birth.
Their findings are published in the journal Nature. The paper’s lead authors are Chenqxiang Qiu and Beth K. Martin of the Department of Genome Sciences at the University of Washington School of Medicine in Seattle and Ian C. Welsh of the Jackson Laboratory in Bar Harbor, Maine.
The scientists wrote that they hope their in-depth sampling will help “advance toward a more comprehensive and continuous view of transcriptional dynamics throughout prenatal development.”
Transcriptional dynamics refers to when cells read and act on plans contained in various parts of their genome. This time-sensitive activity is essential for the production of proteins that guide, for example, the creation and migration of cell types necessary for each stage of embryonic development.
The scientists applied a method called combinatorial optimized single-cell indexing RNA sequencing (sci-RNA-seq) to determine the transcriptional states of cells at intervals of two to six hours, starting at eight days of prenatal development until at birth. Using this information, researchers were able to annotate hundreds of cell types and explore the formation of kidneys, retinas, early neurons, and other tissues.
Combining this latest information with other published data available through open science, the researchers constructed a tree describing the lineages and relationships of cell types throughout mouse prenatal development, from fertilized egg to new -born. Within this tree, they also suggested which genes might be at work in the developing living embryo to drive the emergence of hundreds of cell types.
Immediately after their pups were born, the scientists noticed that massive and abrupt transcriptional changes had occurred in the nuclei of several cell types, such as those in the respiratory tract, liver and fatty tissue. Scientists have hypothesized that these changes may be physiologically necessary because of the profound disruption that occurs between life tied to the placenta and life outside the womb.
A newborn suddenly needs to breathe air, maintain normal sugar levels after being deprived of its mother’s nutrients, and keep its body at the right temperature. However, many other genes whose adaptive function is not yet known shortly after birth have also been discovered. What this means is open to exploration.
According to scientists, the transition from the womb to the outside world is already recognized as “full of physiological perils”.
The researchers added that the kinetic patterns of rapid transcriptional changes during birth were much more complex than initially thought and that some may relate to puppies born vaginally versus those delivered by cesarean section.
In discussing their study, the researchers explained that their goal “was not to learn a specific piece of biology, but rather to advance the foundations of a comprehensive understanding of mammalian development.” They added that the data set examined in this study “is a rich source of hypotheses”, notably suggesting transcription factors that may drive the emergence of all types of prenatal cells.
Researchers also hope that such studies will provide a global framework for studying the genomic basis of mammalian development, perhaps even including postnatal stages. A single-cell timeline of the genetic mechanisms at work throughout mammals’ lives, from conception to extinction, could be the ultimate goal of research of this nature.
More information:
Chengxiang Qiu et al, A single-cell time lapse of mouse prenatal development from gastrula to birth, Nature(2024). DOI: 10.1038/s41586-024-07069-w
Provided by the University of Washington School of Medicine
Quote: Mouse study finds birth coincides with rapid changes in gene activity (February 15, 2024) retrieved February 15, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.