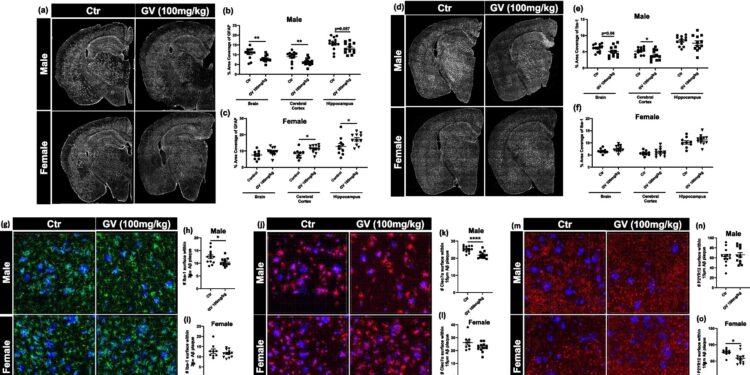
GV-971 significantly reduces glial inflammation in 9-month-old male 5XFAD mice. Credit: Molecular neurodegeneration (2024). DOI: 10.1186/s13024-023-00700-w
According to the Alzheimer’s Association, nearly two-thirds of Americans with Alzheimer’s dementia are women. Although part of this gap can be attributed to the fact that women live longer on average than men, researchers believe biological factors also play a role.
Two new University of Chicago studies explore sex-specific differences in the development of Alzheimer’s-like symptoms in mice, including the impact of estrogen, the primary female reproductive hormone, on amyloid plaque formation and inflammation in the brain. two characteristic symptoms of the disease. The research also strengthens evidence for the role of the gut microbiome in mediating these symptoms, providing clues that could one day help develop treatments.
Clues pointing to the microbiome
Alzheimer’s disease is characterized by the formation of amyloid plaques or clumps of amyloid beta (Aβ) protein that accumulate in the brain. The disease also activates immune cells in the brain called microglia, which can help clear amyloid plaques, but can also exacerbate the disease by causing inflammation.
In 2019, a research team led by Sangram Sisodia, Ph.D., the Sr. Thomas A. Reynolds Family Professor of Neurobiology at UChicago, treated mouse models of Alzheimer’s disease with a cocktail of antibiotics over the course of the second week of life.
Antibiotics reduced amyloid plaque formation and microglia activation in males – but surprisingly not in female mice – at 3 months of age. Although this antibiotic regimen initially eliminates all gut bacteria, the gut repopulates with many additional bacterial species over the next three months.
Sisodia reasoned that the microbiome must play a major role in these changes in amyloid deposition and neuroinflammation. To prove that the improvement in Alzheimer’s symptoms was due to alterations in the gut microbiome, they also transplanted feces from untreated mice into antibiotic-treated animals.
This procedure restored the gut microbiome and caused an increase in amyloid plaque formation and microglial activation. These results have since been confirmed and reported in several laboratories across the country.
Sex-specific microbiome changes
In the first of a new article, published in Molecular neurodegenerationSisodia and colleagues tested the effects of a new drug compound called sodium oligomannate, or GV-971, on the formation of amyloid deposits and neuroinflammation.
The compound was originally derived from brown algae by Chinese pharmaceutical company Shanghai Green Valley Pharmaceuticals. In the company’s testing, GV-971 reduced amyloid deposition and neuroinflammation in mouse models of Alzheimer’s disease. The compound has also undergone phase III clinical trials in China and is now clinically approved for patients with Alzheimer’s disease.
When Sisodia and his team tested GV-971 in a mouse model of Alzheimer’s disease, they found a significant drop in amyloid deposits even at the lowest doses, as well as a reduction in inflammatory markers in the microglia. But again, these changes were only observed in male animals. They also noted significant changes in the composition and abundance of several types of gut bacteria in male mice, but fewer changes in the microbiome of females.
Independently and unbeknownst to Sisodia, David Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor of Neurology at Washington University in St. Louis and co-author of the paper, conducted a similar set of experiences with the GV-971. in a different line of mice and I obtained similar results: levels of amyloid deposition and neuroinflammation were significantly reduced, but only in male mice.
Additionally, a host of bacterial species altered by GV-971 in the Sisodia lab studies also appear to have been altered in Holtzman’s experiments.
Cytoscape and GSEA analyzes using significantly modified genes (P. < 0.01) in the group of men treated with GV-971 compared to the control group. A Cytoscape analysis identified the immunity pathway as a major impacted pathway associated with lower DEGs (P.< 0.01) in GV-971-treated males compared to the control. The most enriched genes are highlighted in darker tones. b Cytoscape analysis identified the neuronal development pathway as a major impacted pathway associated with higher DEGs (P. < 0.01) in the GV-971 treated group compared to the control group. The most enriched genes are highlighted in darker tones. vs GSEA enrichment results showed 9 significantly enriched gene sets out of 44 upregulated gene sets in the vehicle-treated male group, compared to the GV-971-treated male group. Credit: Molecular neurodegeneration (2024). DOI: 10.1186/s13024-023-00700-w
“It was a little crazy because the microbiomes of these mice differ between UChicago and WashU. But ultimately, after we did the treatments, we found out what was in the microbial composition, and there is two or three bacteria that stand out,” Sisodia said. “It’s hard to believe it’s a coincidence, but there must be something to it.”
Sisodia said additional studies are needed to understand the links between GV-971, the microbiome, amyloid deposits and inflammation, either by introducing or removing these key bacteria and analyzing the effects of the metabolites they produce .
“How do these pathways interact? And how does this lead to changes in brain function? All of this remains to be determined,” he said.
Impacts of estrogen
The second study, published in Scientific reports, looked more directly at sex-specific differences in Alzheimer’s disease. In collaboration with the UChicago Microbiome Center, postdoctoral researcher and first author of the study, Piyali Saha, Ph.D., investigated whether circulating estrogen levels could be the reason why female mice do not show reductions amyloid deposits and neuroinflammation after antibiotic treatment.
Saha treated mice with amyloid deposits with antibiotics and measured the levels of estrogen circulating in their blood plasma and found that estrogen levels increased threefold compared to mice treated with saline alone.
Saha reasoned that the increase in estrogen might have something to do with the differences in amyloid deposits seen between antibiotic-treated male and female mice in previous studies. To test this, she conducted a second set of experiments in which she removed the ovaries (called an ovariectomy or OVX) of female mice when they were just a few weeks old, thereby stopping estrogen production.
This procedure reduced both amyloid deposits and inflammatory microglia levels. When another cohort of OVX-treated mice were then given estradiol in their drinking water to restore estrogen levels, amyloid deposits increased again, as did inflammatory microglia. Gut microbiome composition also varied significantly among mice subjected to OVX, those subsequently given estradiol, and controls.
“This came out of nowhere; I had no idea that manipulating estrogen levels was going to change things so dramatically,” Sisodia said. “Estrogen seems to be driving the changes we see in Alzheimer’s pathology, but we also know that the microbiome is changing. So there’s this crosstalk between the two.”
This runs counter to long-standing practice of using hormone replacement therapy to restore estrogen levels in postmenopausal women to help prevent cognitive decline, a strategy called into question by recent epidemiological studies . For example, a large-scale study of more than 20,000 women in Denmark between 2000 and 2018 showed that women who took estrogen replacement therapy had a higher risk of developing Alzheimer’s disease and other diseases. dementia than those who did not receive this treatment.
“This evidence suggests that estrogen replacement therapy is not the right thing to do,” Sisodia said. “We see in the current study that estrogen levels still have an impact on amyloid deposits. If you remove the source of estrogen in mice at a very early stage, the amyloid deposits disappear. That’s quite remarkable .”
Sisodia emphasizes that there is still much to learn about the chain of events that leads from estrogen levels to changes in the gut microbiome and changes in amyloid deposits. It could be that estrogen affects the composition and abundance of certain types of bacteria, which in turn would change the metabolites and enzymes they produce, further impacting brain function.
Timing is also important, because once Alzheimer’s symptoms become apparent, it is far too late to reverse the damage. Completely stopping estrogen production in women is not a solution, but evidence from these studies suggests possible intermediate steps.
“If we can identify some target molecules involved in this biological cascade of estrogen metabolism, we may be able to develop some sort of drug to mitigate the effects,” Sisodia said. “I think it’s potentially an excellent therapeutic avenue, at least for 50% of the population.”
More information:
Megan E. Bosch et al, Sodium Oligomannate Alters the Gut Microbiota, Reduces Brain Amyloidosis and Reactive Microglia in a Sex-Specific Manner, Molecular neurodegeneration(2024). DOI: 10.1186/s13024-023-00700-w
Piyali Saha et al, Early modulation of the gut microbiome by female sex hormones modifies amyloid pathology and microglial function, Scientific reports(2024). DOI: 10.1038/s41598-024-52246-6
Provided by the University of Chicago
Quote: Microbiome studies explore why more women develop Alzheimer’s disease (February 20, 2024) retrieved February 20, 2024 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.