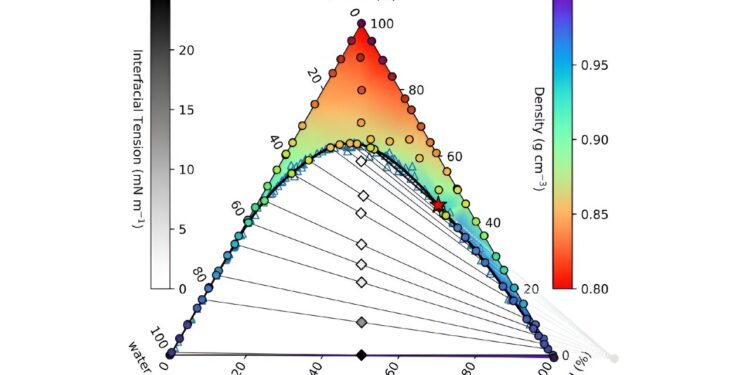
Phase diagram of ouzo. The full figure legend can be found in the corresponding journal article.
Mathematicians at Loughborough University have turned their attention to a fascinating observation that has intrigued scientists and cocktail enthusiasts alike: the mysterious way in which ouzo, a popular anise-flavored liqueur, becomes cloudy when water is added.
The researchers’ exploration of this seemingly simple phenomenon, known as the “Ouzo effect,” has resulted in a new mathematical model that offers insight into how microscopic droplets spontaneously form and how they can remain suspended in a liquid for a long time.
Revealing the mathematics that goes on inside glass could have far-reaching implications beyond the world of beverages, such as the creation of new materials.
“Ouzo is basically three things: alcohol, anise oil and water,” explains Dr. David Sibley, an expert in mathematical modeling.
“When water is added, microscopic droplets form, consisting mainly of oil, and they are the result of the separation of anise oil from the alcohol-water mixture. This makes the drink cloudy because the droplets scatter light.”
He continued: “This emulsification, that is, the suspension of well-mixed oil droplets in the liquid, is something that requires a lot of energy in other systems and foods. For example, food emulsions such as mayonnaise and salad dressings require vigorous whisking to obtain a smooth and stable mixture. For ouzo, on the other hand, the emulsification occurs spontaneously.
“What’s also surprising is how long these droplets and the resulting turbidity remain stable in the mixture without separating, especially when compared to other food emulsions. If you’ve ever made an olive oil and balsamic vinegar vinaigrette, you’ll notice that the two liquids start to separate after a short time, requiring more whisking to bring them together. The ouzo-water emulsion remains stable for a much longer period of time.”
“Understanding how and why this happens in ouzo could lead to the development of new materials, particularly in areas such as pharmaceuticals, cosmetics and food, where the stability and distribution of microscopic particles are essential.”
Loughborough researchers, working with experts from the University of Edinburgh and Nottingham Trent University, have discovered the mathematical principles that explain how droplets and the surrounding liquid – two distinct ‘phases’ within the mixture – form and can remain stable together for long periods.
By mixing alcohol, oil and water in varying proportions, they were able to observe phase separation and measure key properties like surface tension.
They used these data and a statistical mechanical modeling method known as “classical density functional theory” to develop their mathematical model.
This model was used to calculate a phase diagram that details the stable combinations of ouzo ingredients.
The research was published in the journal Soft matter and appears on the cover of the latest issue. The article is titled “Experimental and theoretical volume phase diagram and interfacial tension of ouzo.”
“You could say that what seemed murky is now clearer,” said Professor Andrew Archer, first author of the paper.
“What’s also fun is that simple models like this can predict a lot of things, like recent parallel research we’ve done that reveals how long the droplets we sneeze in the air can linger.
“As is often the case, basic research on ‘blue sky’ can reveal something profound about an experience that occurs in everyday life, such as serving and drinking ouzo.”
More information:
Andrew J. Archer et al, Experimental and theoretical volume phase diagram and interfacial tension of ouzo, Soft matter (2024). DOI: 10.1039/D4SM00332B
Provided by Loughborough University
Quote:Mathematicians unlock secrets of ouzo’s murky transformation (2024, August 22) retrieved August 22, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.