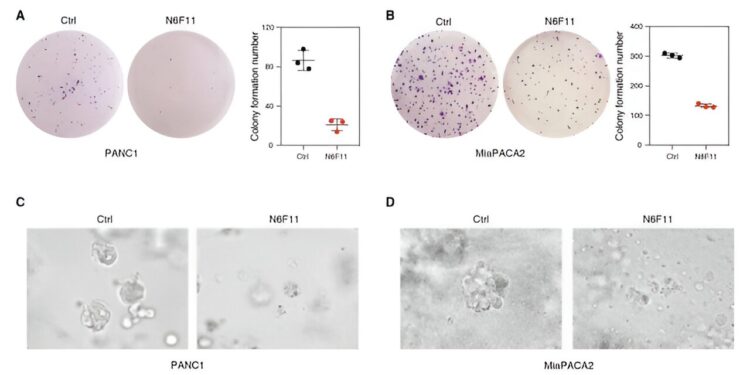
N6F11 suppresses human PDAC growth. (A) PANC1 cells were pretreated with DMSO or N6F11 (5 µM) for 12 hours. (B) MiaPACA2 cells were pretreated with DMSO or N6F11 (5 µM) for 12 hours. (C) Effects of N6F11 on the growth of PANC1 3D tumor spheroids for increasing periods. (D) Effects of N6F11 on the growth of MiaPACA2 3D tumor spheroids for increasing periods of time. Credit: Scientific translational medicine (2023). DOI: 10.1126/scitranslmed.adg3049
Scientists have discovered an experimental small molecule that induces a form of cell death to kill various cancers while boosting the power of the immune system and leaving healthy cells completely unharmed.
The molecule triggers ferroptosis, a unique form of cell death that is increasingly being tested as an anticancer strategy. The international team of scientists published their findings in the journal Scientific translational medicine.
For biology students, the three main forms of cell death are well known and taught as early as the first cycle of undergraduate general biology courses. This is apoptosis, or type 1 cell death; autophagy, type 2 cell death; and cell necrosis, or type 3. Ferroptosis, on the other hand, is a distinct form of cell death that relies on an accumulation of iron and the generation of reactive oxygen species, which ultimately causes the self-destruction of a condemned cell.
It is vital to turn to an alternative form of cell death, as most forms of cancer treatment today trigger cell death through enzyme-dependent apoptosis. Unfortunately, one of the main hallmarks of human cancers is their ability to develop resistance to treatment, and many tumor types have shockingly developed resistance to apoptosis, hence the search for another way to kill tumor cells.
As intriguing as ferroptosis may seem as an alternative, it is not yet ready to be exploited as a formal therapeutic to treat cancers in humans. But just because it’s not ready for prime time yet doesn’t mean it won’t be in the not-too-distant future.
“We believe that such an approach warrants further preclinical evaluation for possible incorporation into the oncologic armamentarium,” writes lead author Dr. Jingbo Li, a researcher in the Department of Surgery at Southwestern Medical Center from the University of Texas in Dallas, Texas. Li is also affiliated with the Department of Gastroenterology at Xiangya Hospital of Central South University in Changsha, China.
Although ferroptosis is actively researched as a potential cancer treatment, other researchers study the process for its pathological role in a variety of disparate diseases ranging from Alzheimer’s disease to cardiovascular disease and even various forms of cancer. Ferroptosis is intimately involved in the pathological processes of these conditions, studies have shown.
When it comes to harnessing this form of cell death for therapeutic purposes, teams around the world are working to overcome a number of conundrums, particularly the so-called “non-selective” activities of ferroptotic cell death. It doesn’t just kill cancer cells, it kills a multitude of cells in the immediate microenvironment, particularly the big three: dendritic cells, T cells and neutrophils, which seems to thwart the promise that ferroptosis holds in as a cancer fighter.
This means that most compounds capable of inducing ferroptosis in cancer cells can also inflict the same fate on various immune cells, weakening the immune system’s ability to intervene and wage war against deadly tumors.
Now, Li and a team of collaborators on three continents have identified a promising small molecule called N6F11, which not only triggers ferroptotic cell death but also selectively causes the degradation of glutathione peroxidase-4, also known as GPX4, a notorious agent. ferroptosis blocker.
Li and his colleagues, who embarked on an extensive drug hunt, screening a large number of compounds to find N6F11 and its unique properties, say that with N6F11 in the mix, ferroptosis can be triggered and GPX4 does not is better able to prevent this specialized phenomenon. form of cell death due to the annihilation of tumors. Even more telling, N6F11 degraded GPX4 in human pancreatic, bladder, breast, and cervical cancer cells without affecting GPX4 in this vital immune system trio: dendritic cells, T lymphocytes, and neutrophils.
Li, along with colleagues at Columbia University in New York, the University of Paris in France, a large team at UT Southwestern Medical Center in Dallas and beyond, also found that N6F11 slowed the growth of active tumors in mouse models inoculated with pancreatic cancer cells. The animals endured the treatment without serious side effects, an effect the authors linked to N6F11’s ability to stimulate T cells.
“Lipid peroxidation-dependent ferroptosis has become an emerging strategy for tumor treatment,” Li added. “However, current strategies not only selectively induce ferroptosis in malignant cells, but also simultaneously trigger ferroptosis in malignant cells. immune system, which can compromise anti-tumor immunity.”
Li explained in Scientific translational medicine that using In-Cell Western assays, combined with massive drug screening, the team was able to identify and confirm the compound N6F11 as a ferroptosis inducer.
“An effective antitumor adaptive immune response requires dendritic cells that present tumor-associated antigens to T cells,” Li continued. “Initiation of the anticancer response by dendritic cells depends largely on sufficient immune signals, in particularly molecular patterns associated with pathogens and molecular patterns associated with damage.
“Induction of immunogenic cell death by chemotherapy, radiotherapy or targeted therapies can trigger an adaptive immune response by exposing and releasing a variety of damage-associated molecular patterns.
“However, it is unclear which type of cell death optimally enhances the immunogenicity of tumor cells,” Li added, noting that ferroptotic cell death depends on unrestricted lipid peroxidation rather than the cascade of enzymatic events associated with apoptosis.
“In summary, we identified a small molecule, N6F11, that induces the selective degradation of GPX4 in malignant, but not immune, cells,” concluded Li. “In small tumors, N6F11-induced ferroptosis (and) initiates a powerful anti-tumor immune system.”
More information:
Jingbo Li et al, Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer, Scientific translational medicine (2023). DOI: 10.1126/scitranslmed.adg3049
© 2023 Science X Network
Quote: Massive drug search discovers infinitesimal molecule that kills cancers while sparing immune cells (December 19, 2023) retrieved December 20, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.