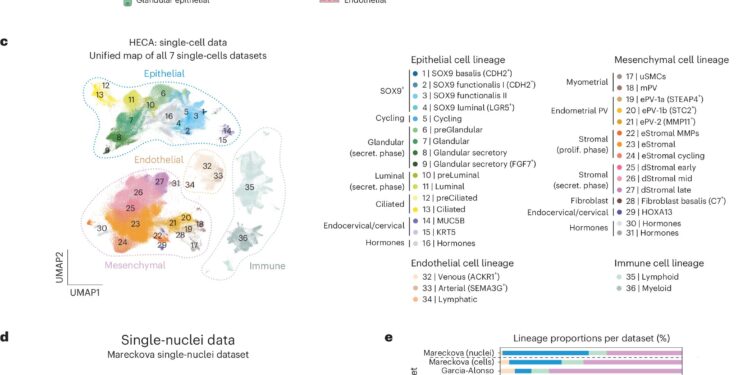
Harmonized cellular map of the human endometrium. Credit: Genetics of nature (2024). DOI: 10.1038/s41588-024-01873-w
The most comprehensive map of the human endometrium, the inner lining of the uterus, has been created, revealing diverse cell types and detailing the dynamic changes they undergo during the menstrual cycle.
Covering the widest range of menstrual cycle phases ever mapped, the atlas reveals new insights into how the endometrium functions, informing research into women’s health. Developed by researchers from the Wellcome Sanger Institute, the Nuffield Department of Women’s and Reproductive Health at the University of Oxford and collaborators, it could help to study, understand and potentially treat diseases such as endometriosis in the future.
The study, published in Genetics of natureis part of the larger Human Cell Atlas project, which maps every cell type in the body to transform understanding of health and disease.
By linking it to genetic variants known to increase the risk of endometriosis, the researchers discovered two types of immune cells and two types of stromal cells potentially involved in the disease, highlighting new avenues for future research.
The Human Endometrial Cell Atlas is now publicly available in an easy-to-access interactive format and is expected to be a valuable central resource for researchers studying the endometrium and also be used to inform and develop more effective laboratory models.
Having a detailed map of the endometrium allows researchers to gain insight into unique cells and interactions not found elsewhere in the body, and to better understand tissue changes during the menstrual cycle, including the endometrium’s ability to regenerate without scarring.
The endometrium is essential for human reproduction, as it promotes pregnancy if a fertilized egg is implanted. If implantation does not occur, it sheds and rebuilds itself each month without leaving scars. This ever-changing tissue undergoes complex and dynamic changes throughout the menstrual cycle, making it extremely difficult to study.
Endometrial diseases affect millions of people worldwide. Endometriosis, a chronic condition in which endometrial-like cells grow outside the uterus, is the second most common gynaecological disease in the UK. Among other symptoms, it can cause debilitating pain and fertility problems. There is currently no cure and the cause of the disease is unknown.
Previous studies of the human endometrium and uterus have been able to provide a limited picture, but never one that captures all of the stages the endometrium goes through during the menstrual cycle.
This new atlas of endometrial cells, led by researchers from the Wellcome Sanger Institute and the Nuffield Department of Women’s and Reproductive Health at the University of Oxford, generated new data on 74 endometrial samples. They matched the data with existing single-cell data from 47 people.
In total, the atlas contains data on approximately 626,000 cells from 121 people, including individuals with and without endometriosis, both during natural menstrual cycles and while taking hormonal contraception.
The team discovered several new cell types that are only present in certain phases of the menstrual cycle, depending on hormone levels. A cellular response to hormone levels is essential for menstrual cycle progression and fertility, and these cells could be promising therapeutic targets for pathologies related to hormonal disruption, such as fertility disorders.
Researchers have discovered interactions involved in scarless endometrial regeneration between immune cells called macrophages, a type of connective tissue cell known as stromal cells, and blood vessel cells. Understanding how these pathways can be disrupted in common menstrual conditions, such as abnormal bleeding where the lining continues to shed, could help find new interventions.
Although there were no notable differences in the number of cell types between women with and without endometriosis, there were small differences in the proportion and gene expression of some cells in women with endometriosis. These findings are consistent with previous work, but the atlas provides a more detailed view of the specific cell types that may be dysregulated in endometriosis.
To study the impact of genetic variants that have already been linked to endometriosis, the researchers combined their in-depth cellular map of the endometrium with a large genome-wide association study.
The team identified four cell types that are likely deregulated by these genetic changes. They also found that specific signaling pathways between certain stromal cells and structural cells were deregulated in people with endometriosis. These signaling pathways are known to be necessary for the progression of the menstrual cycle.
The study highlights that these cell types and signaling pathways could be involved in endometriosis and could support future research examining how genetic changes are linked to this condition.
“Having this deep, large-scale genomic resource on the endometrium is invaluable if we are ever to fully understand how the endometrium functions in health and what goes wrong in conditions such as endometriosis.
“Developing a non-invasive diagnostic test and effective treatment for this debilitating disease has been a priority for clinicians, researchers and people with endometriosis around the world.
“Although further research and validation are needed, our study suggests that certain cells and pathways are deregulated in endometriosis and if replicated in further studies, could be potential diagnostic and therapeutic targets in the future,” says Dr. Magda Marečková.
“Creating an integrated atlas of human endometrial cells transforms all data into a ‘single language’ that researchers around the world can speak. We hope this atlas will be a key step toward building a future endometrial atlas that includes information across the lifespan and all health conditions.”
“Further studies that collect additional health information, such as whether the person has regular periods or a previous pregnancy, as well as genomic data could help build the atlas and further study factors that could play a role in uterine health and the development of endometrial diseases,” says Dr. Luz Garcia-Alonso.
“The human endometrium has been largely neglected in large-scale cellular studies of different parts of the body. Having a large, freely available, and continuously expanded, single-cell human endometrial atlas will enable important new research in understanding and treating diseases specific to women and people born with a uterus, such as endometriosis,” says Professor Krina Zondervan.
“The human uterus is a dynamic and poorly understood part of the body, containing key information that could be used to treat a range of diseases. Understanding the impact of hormones on the endometrium and being able to map all the changes throughout the menstrual cycle is something that was not previously possible and would not have been possible without those who have generously donated endometrial tissue to research.
“We were able to reveal unique interactions and cells that can inform research models, investigate possible causes of disease, and create a resource that can be freely used around the world to help develop new therapies for people living with endometrial diseases,” says Dr. Roser Vento-Tormo.
More information:
Magda Marečková et al., An integrated single-cell reference atlas of the human endometrium, Genetics of nature (2024). DOI: 10.1038/s41588-024-01873-w
Provided by the Wellcome Trust Sanger Institute
Quote: A map of the human endometrium reveals hidden health clues (2024, August 28) retrieved August 28, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.