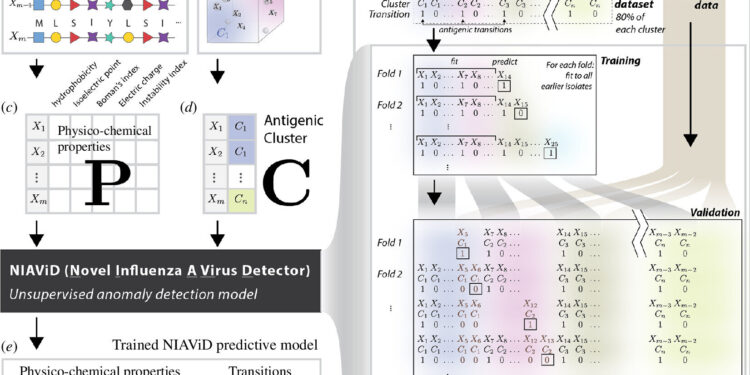
NIAViD is a model for detecting antigenic transitions in influenza A (H3N2) without knowledge of group identity. This figure illustrates the computational flow used to fit and validate NIAViD. Credit: Proceedings of the Royal Society B: Biological Sciences (2024). DOI: 10.1098/rspb.2024.0790
A team led by scientists from UGA’s Odum School of Ecology has developed an algorithm that can accurately predict how a seasonal flu virus will evolve. This information could help update seasonal flu vaccines more quickly, leading to fewer infections and deaths.
Published in the journal Proceedings of the Royal Society B: Biological SciencesThe study describes a machine learning tool called Novel Influenza Virus A Detector (NIAViD) that predicts changes in seasonal flu viruses with nearly 73% accuracy. Alpha Forna, a postdoctoral research associate at the Odum School, led the development of NIAViD, which uses influenza virus sequence data to understand how emerging viruses are expected to circumvent existing immunity in a population.
Each year, seasonal influenza viruses infect 1 billion people worldwide, with the majority of the estimated 500,000 deaths occurring among young children in developing countries. In the United States, millions of Americans are infected each year and thousands die. The estimated annualized cost of seasonal infections is approximately $90 billion in the United States alone.
According to Forna, one of the challenges in preventing seasonal influenza infections is predicting the expected evolution of the influenza virus.
“Every influenza virus, which we call influenza, has a hemagglutinin protein that triggers infection when it attaches to cells. During an infection, influenza viruses begin to replicate and at that point small genetic changes in the hemagglutinin protein occur, which we call antigenic drift,” he said.
“Some of these changes can make the flu virus unrecognizable to our immune system. This means that even if a person has already been infected with the flu or received a vaccine, they can still be infected with a flu virus that has a slightly changed hemagglutinin protein.”
Machine learning, a method of programming computers to learn on their own, is an increasingly important tool in infectious disease modeling research. Forna spent two years building the NIAViD system, accessing and interpreting large data sets and training models to make accurate predictions. He and his colleagues developed a specific set of algorithms designed to analyze amino acid sequences in a region of the hemagglutinin protein gene and several associated properties that quantify features, such as a virus’s electrostatic charge.
After training on a subset of the data, the model achieved an accuracy rate of nearly 73%, matching or exceeding the performance of other models. By focusing on key properties, NIAViD accurately identifies antigenic changes, helping to rapidly update influenza vaccines.
NIAViD was developed around an influenza virus that first emerged during the 1968 pandemic that began in Hong Kong. After that virus began circulating in the global human population, labs around the world began testing the collected viruses to understand how they responded to antibodies that were known to protect people from infection. As viruses that were immune to antibodies or vaccines were discovered, groups were eventually identified, leading to early predictive modeling that helped vaccine makers focus their efforts on emerging influenza lineages.
Co-author Justin Bahl, a professor in the UGA School of Veterinary Medicine, said those early research projects were instrumental in vaccine development.
“Grouping influenza viruses into specific groups to understand their level of immunity to vaccines was innovative,” he said. “However, this research was based on a virus surveillance methodology that is labor-intensive, time-consuming and can be unreliable. We believed that a faster and more accurate method of predicting emerging seasonal influenza viruses could be implemented using an infectious disease machine learning algorithm.”
The success of NIAViD opens up many avenues for future research, according to Professor John Drake, director of the Center for Infectious Disease Ecology.
“Its ability to rapidly identify antigenic variants can support ongoing influenza vaccine development, enhancing public health preparedness and responsiveness to seasonal influenza,” he said. “Integrating NIAViD into surveillance systems would allow manufacturers to stay ahead of viral evolution, ensuring that vaccines target the most recent strains.”
More information:
Alpha Forna et al, Sequential detection of novel antigenically emerging influenza A viruses, Proceedings of the Royal Society B: Biological Sciences (2024). DOI: 10.1098/rspb.2024.0790
Provided by the University of Georgia
Quote:Machine learning could lead to better flu vaccines (2024, September 9) retrieved September 9, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.