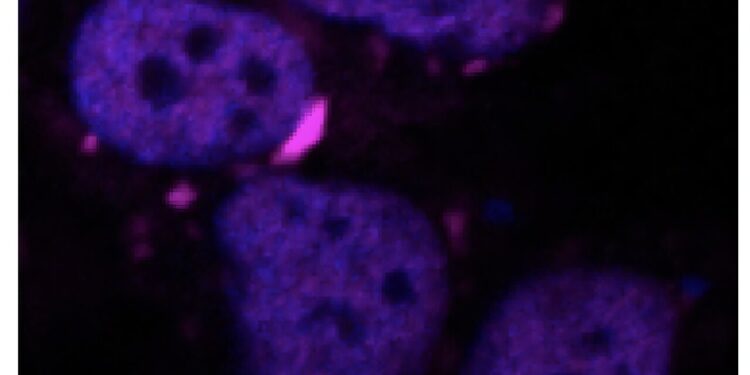
Example of protein aggregates in a cell. Credit: Iit-isito Italiano di Tecnologia
A research group led by Gian Gaetano Tartaglia, principal researcher from the Italian Technology Institute (IIT), has developed a machine learning algorithm to study the behavior of proteins within cells and to predict their ability to trigger neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Parkinson and Alzheimer.
The algorithm, called Catgranule 2.0 robot, helps to identify molecular targets for new research and therapies. It is described in an article published in the journal Genome biology.
Neurodegenerative diseases are a major health challenge with major socio-economic results: in Italy, around 1 million people are affected and a typical patient costs around 70,000 euros on average. The group led by Tartaglia studies the chemical-physics behavior of certain proteins linked to the beginning of these diseases.
In the cellular environment, these proteins have the capacity to create biomolecular condensates, very tangled tufts which, under certain conditions, become insoluble in water.
In an unassigned organism, this process manages the production of other proteins and responds to cellular stress, but an impairment of the condensation mechanism can cause a pathological state: piles of protein become solid structures that accumulate in cells and cause their death. Examples of these toxic aggregates are Lewy’s bodies in Parkinson, the accumulation of filaments in the motor neuron linked to SLA and amyloid plates linked to Alzheimer.
The transition of a healthy situation to a disease is often due to changes in the structure of proteins: a new structure can transform biomolecular condensates into solid aggregates.
Under the supervision of Tartaglia, Iit Post-Docs Michele Monti and Jonathan Fiorentino have developed a Catgranule 2.0 robot (acronym for the organization of ribonucleoprotein in the types of biocondensate organels). This automatic learning tool explores the link between changes in protein structures and the formation of condensates.
“The identification of biochemical signals linked to neurodegenerative diseases is crucial for early interventions and to slow down the cognitive decline”, explains the research coordinator Gaetano Tartaglia. “We have trained our system to recognize the formation of condensates, which, in many cases, is an initial step for the formation of toxic aggregates. A key contribution to this event is the protein-arn interaction.”
The physical chemical mechanism which leads to the formation of biomolecular condensates is the separation of liquid-liquid phase and certain proteins have a three-dimensional structure adapted to promote this process. RNA also regulates the formation of these tufts: its interaction with proteins facilitates or inhibits the separation of the phases.
The research group has focused its attention on the PRN-Protein interaction and formed the Catgranle 2.0 robot to use this parameter to determine whether a biomolecular condensate has the potential to occur.
The automatic learning algorithm studies the structure of a protein, analyzing its amino acid sequences and considering its affinity for RNA. Thanks to this analysis, researchers can determine if the protein could generate toxic condensates when it undergoes phase separation.
With the robot method, the researchers study the influence of mutations on the liquid-liquid separation: if a change of structure modifies the protein-arn interaction, this can cause pathological consequences, because it modifies the formation of condensates.
This study is carried out within the framework of the IVBM-4PAP project, coordinated by the Italian Institute of Technology, which aims to develop the Brillouin in Vivo (IVBM) microscope, a tool to identify new therapeutic targets for the treatment of neurodegenerative diseases.
IVBM will measure the properties of proteins and condensates within living cells, without external interventions. The Catgranule 2.0 robot is the calculation basis on which the research consortium develops the project: it provides theoretical predictions on which proteins and mutations could be relevant.
Researchers will use the microscope to check the calculations, the cell observation, the real -time behavior of the proteins and their interaction with the RNA.
Integrate the work of calculating algorithm with the experimental activities implemented under the microscope, researchers have a tool to identify early pathological signals and to develop new therapeutic strategies that slow down the progression of neurodegenerative diseases. This strategy could help reduce their long -term impacts.
The Consortium IVBM-4PAP is made up of the Center for Life Nano and Neuro-Science and the RNA Systems Biology Lab of the Italian Institute of Technology, the University of Trento, Universidad Zaragoza, the Imhorphen group of Angers University and Crest Optics.
More information:
Michele Monti et al, Catgranule 2.0: Precise predictions of liquid-liquid phase separation proteins to a single amino acid resolution, Genome biology (2025). DOI: 10.1186 / S13059-025-03497-7
Provided by the Italian Institute of Technology
Quote: Hunting Algorithm Automatic learning for proteins damaging the brain (2025, April 2) recovered on April 2, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.