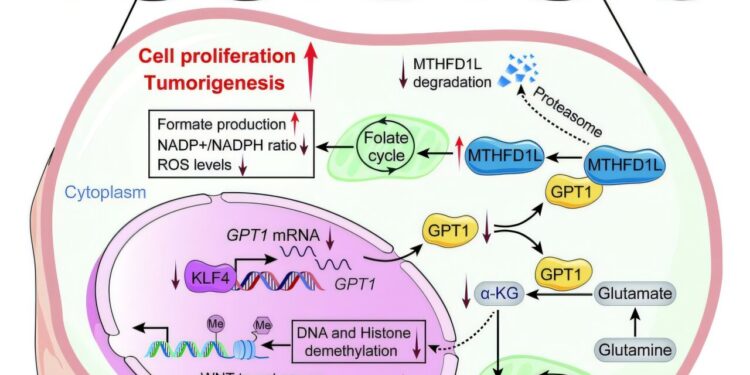
Schematic model illustrating the mechanisms of GPT1 in CRC tumorigernesis. Credit: Translational scientific medicine (2025). DOI: 10.1126 / Scitranslmed.adp9805
The transformation of healthy cells into invasive colorectal tumors is an extraordinarily complex process involving many molecular mechanisms, according to cancer biologists in China who discovered that low levels of a single enzyme strongly influence the way of malignancy.
Scientists from around the world have been looking for neglected and underestimated biological paths involved in the tumorigerosis of one of the most common forms of cancer. The team of researchers in China who discovered the last underlines that maybe others remain to be found.
Colorectal cancer – CRC – is the third form of cancer around the world, and although generally associated with more advanced age, the disease inexplicably increases in people under the age of 50, a particularly obvious trend in the United States. A study of 2023 American Cancer Society revealed that 20% of CRC diagnoses in 2019 involved people under 55, double the rate of 1995. In the same study, advanced disease rates increased by 3% in people under 50.
Whatever the age when cancer is diagnosed, oncologists say that the most common risk factors are physical inactivity; obesity; low consumption of dietary fiber; smoking; Family history of CRC or Colon polyps; Inflammatory intestine disease; Certain genetic conditions, such as Lynch syndrome and excessive alcohol consumption.
Scientists from the Colorectal Surgery Department of the Sixth Affiliate Hospital of the Sun Yat-Sen University of Guangdong examined how CRC emerges. By working with collaborators elsewhere in China, they found that a critical enzymatic imbalance can stimulate the formation of tumors in colorectal cancer.
Report in Translational scientific medicineThe team has highlighted an enzyme called GPT1, which means glutamic-pyruvic transaminase 1. Colorectal cancer, affirmed by researchers, is characterized by a reduction in the quantities of GPT1, a metabolic enzyme with apparent functions in the progression of cancer.
The main author of the study, Li Xiong, as well as a large team of co-investigators, confirmed a decrease in GPT1 in CRC patients and have demonstrated in a series of experiences that a low expression of GPT1 has been associated with worse prognosis of CRC. The researchers were able to define the role of GPT1 in colorectal cancer by tracing the progression of normal cell disease with the precancerous cell to a full -fledged malignant tumor. The team was able to deduce the importance of GPT1 in the CRC by noting its decreasing levels as the disease progressed.
“Colorectal cancer tumorigesis – CRC – follows the normal adenoma carcinoma factory – NAC – sequence”, writes Xiong in the study.
Even if the molecular mechanisms underlying colorectal carcinogenesis of the adenoma remain largely unknown, the Guangdong team has been able to define a causal role for the low GPT1. “We have analyzed transcriptomic profile changes in normal and advanced adenoma tissues and carcinoma of CRCs,” added Xiong, noting that as the disease progresses, GPT1 is regulated downwards, which means the enzyme and, consequently, its activity has radically decreased.
The study also revealed that a compound of the name of politicians, which activates GPT1, can remove tumor growth, which suggests that poliiumoside could be translated into new therapy for CRC, allowing a potentially new method of tumor deletion.
As the third most common form of cancer, CRC at advanced stages still has poor survival rate, a factor that highlights the need for new treatments. Because these tumors develop from precancerous adenomas in the lining of the colon, medical investigators, such as Xiong and its colleagues, have looked for current molecular changes between precancerous and cancerous stages.
It is well known that adenomas accumulate genetic mutations and undergo changes in metabolism which destabilize them along the way of malignancy. However, scientists have still not identified all the molecular mechanisms that shape the transformational path to CRC. The identification of GPT1 is a step towards training one of the multiple stages involved in the tumorigesis of colorectal cancer.
“About 85% of CRCs arise from adenomas, and advanced adenomas are considered the main precancerous lesions leading to colorectal carcinogenesis,” explained Xiong in the study. “An increased rate of adenoma detection is associated with a reduction in the risk and mortality of the CRC, stressing the importance of early detection and the elimination of precancerous lesions.”
Xiong stressed that some patients who have had kidnapped adenomas still have an increased risk of developing new adenomas or CRCs. To monitor the growth of adenoma, the American multi-society working group on CRC and the European Gastrointestinal endoscopy company recommend monitoring colonoscopy three years after the elimination of an advanced adenoma equal to or greater than 10 millimeters. Adenomas of this size suggest a potentially high risk of CRC development.
“However, effective indicators to precisely assess the risk of adenoma transformation still lacks,” said Xiong, “making it difficult to make sure that high -risk patients receive rapid intervention and low -risk patients avoid useless colonoscopy.
To better understand the role of GPT1, the Guangdong team examined normal colorectal tissues, adenomas and tumor tissues of patients with colorectal cancer. The samples have shown a marked lack of GPT1, the deficiency of which was also correlated with lower clinical results. By studying cells and animal models in the laboratory, the authors then showed that GPT1 normally suppressed the formation of adenoma tumors by producing a metabolic molecule called α-Ceteoglutarate, which inhibited the WNT signaling pathway, disturbing the metabolically important folate cycle.
Regarding the police, the team of cancer biologists found that it reactivated GPT1 and slows down the growth of tumors. Tumor growth has been slowed down in organoids derived from the patient and colorectal cancer mouse models, indicating that the compound should be tested in clinical trials.
“In this study, we identified GPT1 as a regulator involved in metabolic reprogramming and initiation and progression of CRC,” concluded Xiong. “GPT1 deficiency has favored CRC tumorigerisse by recashing cell metabolism in the manner dependent on enzymes and independent of enzymes.”
More information:
Li Xiong et al, metabolic reprogramming mediated by glutamic-pyruvic impairment, facilitates the colorectal progression of the adenoma-carcinoma, Translational scientific medicine (2025). DOI: 10.1126 / Scitranslmed.adp9805
© 2025 Science X Network
Quote: Low levels of a single enzyme influence the path of malignancy in colorectal cancer, scientists find (2025, April 1) recovered on April 2, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.