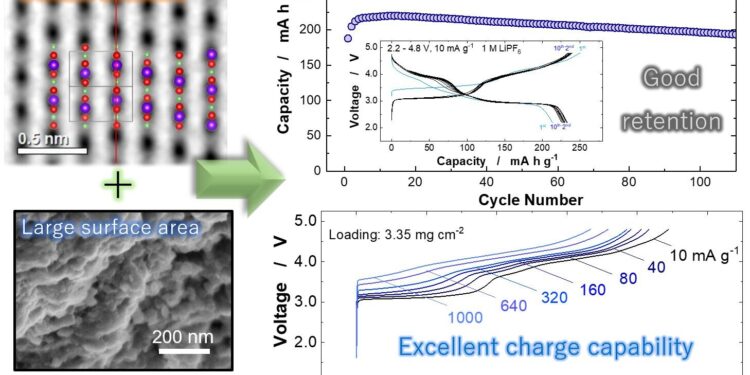
Nanostructured LiMnO2 With domain structures and larger surface area, it provides high reversible capacity with good capacity retention and excellent charge rate capability, which is an essential character for electric vehicle applications. Credit: Yokohama National University
Lithium-ion (or Li-ion) batteries are heavyweights in the world of rechargeable batteries. As electric vehicles become more common around the world, a high-energy, low-cost battery that utilizes the abundance of manganese (Mn) may be a sustainable option to become commercially available and used in the automotive industry.
Currently, batteries used to power electric vehicles (EVs) are based on nickel (Ni) and cobalt (Co), which can be expensive and unsustainable for a society where the desire for EVs is growing.
By replacing the positive electrode materials with a lithium/manganese-based material, the researchers aim to maintain the high performance of Ni/Co-based materials but with a sustainable and low-cost solution.
The researchers published their results in Central Scientific Center of the ACS August 26, 2024.
Li-ion batteries are not new players in the field of rechargeable electronics, but there are always ways to innovate and improve upon already reliable methods. LiMnO2 As an electrode material, it has been studied in the past, but has always been limited by the restrictive performance of electrodes.
“Thanks to the systematic study of different LiMnO2 polymorphs, it was found that the monoclinic layered domain efficiently activates the structural transition to the spinel-like phase. From this discovery, the nanostructured LiMnO2 “With the monoclinic layered domain structures and large specific surface area, it was directly synthesized using a simple solid-state reaction,” said Naoaki Yabuuchi, author and researcher of the study.
A monoclinic system refers to the type of group symmetry of a solid crystal structure. A Li/Mn arrangement with monoclinic symmetry seems to be the key to making LiMnO2 a possible option for a positive electrode material.
Without the structural phase transition enabled by the monoclinic domain, the electrode performance would be limited due to the suboptimal crystal structure of LiMnO2 and the phase transitions that accompany them.
After observing and testing the different polymorphs, it was determined that the required structure can be synthesized directly from two components without the need for an intermediate step. The resulting material is competitive with nickel-based layered materials and has excellent fast-charging capabilities, which are essential for electric vehicles.
Nanostructured LiMnO2 with the monoclinic layered domain is synthesized by a simple calcination process to give a product with a high energy density, reaching 820 watt-hours per kilogram (Wh kg-1), compared to approximately 750 Wh kg-1 for nickel-based laminated materials and 500 Wh kg-1 for other low-cost lithium-based materials.
No voltage decay was reported using nanostructured LiMnO.2which is common in manganese-based materials.
Voltage decay is a phenomenon in which voltage gradually decreases, reducing the performance and responsiveness of electronics over time. However, this does not appear to be an observable problem in the case of nanostructured LiMnO2which is the subject of the study.
Although the results are promising, a practical problem can be observed: manganese dissolution. Over time, manganese can dissolve due to many factors, such as phase changes or reaction with acidic solutions. Fortunately, this phenomenon can be limited or completely mitigated by using a highly concentrated electrolyte solution and a lithium phosphate coating.
The researchers hope their findings will contribute to a more sustainable energy source than fossil fuels, particularly when it comes to electric vehicles.
LiMnO performance2with its competitive energy density compared to nickel-based materials, demonstrates the potential that alternative materials can have to produce environmentally friendly products that are sustainable both in production and as a long-term investment.
An ideal future for nanostructured LiMnO2Silicon-based electrode materials could be commercialized and industrially produced in the luxury electric vehicle industry.
More information:
A practical and durable Ni/Co-free high-energy electrode material: nanostructured LiMnO2, Central Scientific Center of the ACS (2024). DOI: 10.1021/acscentsci.4c00578
Provided by Yokohama National University
Quote:LiMnO₂ electrodes could replace Ni/Co in electric vehicle batteries (2024, August 26) retrieved August 26, 2024 from
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.