Researchers have identified how the presence of residual aggregates can influence supramolecular chirality, opening new avenues in electronic materials. Credit: Takuho Saito / Chiba University, Japan
Self-assembly or self-organization in molecular science refers to the phenomena where molecules gather spontaneously and form ordered structures, a unique property of materials used to develop optical and electronic materials.
In a step to the fine adjustment of this property, the researchers from Japan managed to elucidate a technique where a small amount of residual aggregates have considerably changed the process of self-assembly of the photo-replied molecules.
The research team was led by Professor Shiki Yagai of the Graduate School of Engineering of the University of Chiba, including the assistant professor Takuho Saito of the University of Nagoya (at the time of research), Mr. Daisuke Inoe and the assistant professor Yuichi Kitamoto of Tohoku University, as major contributors to this work.
The results of their study were published online in Nanotechnology of nature.
In recent years, there has been an increasing objective in research on size control and hierarchical structures of self-assembled aggregates, which could help reach aggregates with desired properties. However, self-assembly is a dynamic process and requires specific control.
“During the self-assembly process, the molecules continue to associate and dissociate themselves on several occasions,” explains Professor Yagai, “even tiny impurities or light changes under the conditions can have an impact on the final structure of the formed aggregates”.
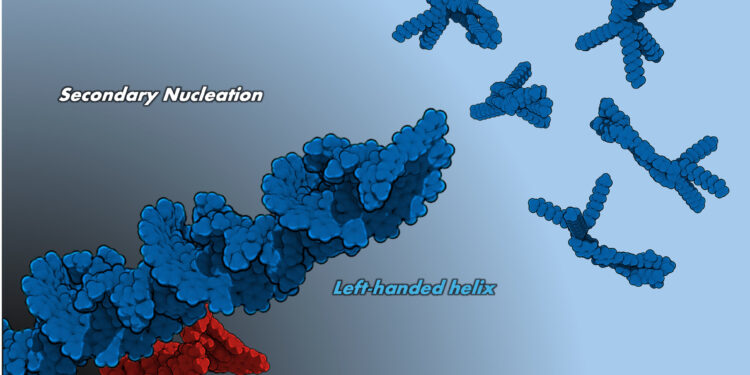
For the study, the research team focused on the self-assembly of a chiral and photooresensive azobenzene which naturally forms left-wing helical aggregates. The team discovered that the presence of a small amount of residual aggregates within the solution induces a radical change in the assembly process and leads to the formation of right -handed helical aggregates instead.
In addition, being photore, control of exposure to light also modifies the time of molecular assembly. Using a precise control of these two properties together, the researchers managed to manipulate the formation of helicoids left -handed or right -handed aggregates if necessary.
In spectroscopic and molecular modeling studies, the team noted that when the chisel -shaped azobenzene molecule is dissolved in an organic solvent at room temperature, it forms a folded structure of the closed scissors which still extends in a helical assembly.
Professor Yagai explains the left assembly formation, saying: “The molecule contains an atom of carbon which has four different atomic groups and therefore has chirality. These molecules bend like left -handed scissors and twist to form a left helical stack of assembly.”
Since these are photooressive molecules, when the stacked helicoidal structures are exposed to a low ultraviolet light (UV), the helicoid assembly is restored in the individual molecules and during a later exposure to the visible light, the molecules are recovered again in the helicoidal structures.
Interestingly, under certain conditions, the resulting helical aggregates proved to the right instead of left -handers, and an exposure to a stronger UV light followed by visible light led to the regeneration of helical aggregates from the left to the left.
By studying closely this mechanism, the team noted that when the solutions were exposed to a small UV light, there were a minute of residual left -handed helicopter aggregates which remained unchanged, and these aggregates acted as nucleation sites forming helicoidal assemblies led by opposite.
“This remarkable phenomenon is called” secondary nucleation “, which explains why the aggregates of meta-stable right-handers are preferably formed instead of left-handed aggregates,” explains Professor Yagai.
In addition to this, the team also discovered the role of the intensity of light in the molecular assembly process.
Professor Yagai explains: “We have identified that the intensity of visible light has potentially affected the assembly calendar. A strong visible light favored a quick assembly while minimizing the influence of residual aggregates. On the other hand, a lower intensity enlarges the effect of residual aggregates.”
Consequently, by optimizing the intensities of UV and visible light, the researchers succeeded in controlling the switching between the left and right helical structures, which depended on the influence of the residual aggregates.
In addition, it has also been found that stable aggregates on the left and meta-stable right-handers also have an opposite electronic spin polarization, which means adjusting the electronic characteristics of the propellers.
Overall, this study aimed to explore the critical role of residual aggregates and explained how the fine adjustment can lead to the manufacture of new functional materials, giving promising information on the field of materials science.
More information:
Inversion of supramolecular chirality by secondary nucleation photographed, Nanotechnology of nature (2025). DOI: 10.1038 / S41565-025-01882-8
Supplied by Chiba University
Quote: Illuminating torsion: light inversion of supramolecular chirality (2025, April 11) recovered on April 11, 2025 from
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.