Top: Intestinal stem cells lacking Daam1 (left) or Rnf43 (right) form tumor-like organoids. Credit: Gabriele Colozza/IMBA
Stem cells can differentiate to replace dead and damaged cells. But how do stem cells decide what type of cell to become in a given situation? Using intestinal organoids, Bon-Kyoung Koo’s group from IMBA and the Institute for Basic Sciences identified a new gene, Daam1, which plays a critical role in triggering the development of secretory cells in the ‘intestine.
This observation, published on November 24 in Scientists progressopens new perspectives in cancer research.
Our body is, in some ways, like a car: to keep it running, it needs to be checked and repaired regularly. In the case of our body, any damaged or dead cells must be replaced for the organs to continue functioning. This replacement occurs thanks to adult stem cells residing in the tissues.
Unlike embryonic stem cells, which can form any type of cell in the body, adult stem cells will only form the cell types found in the tissues to which they belong. But how do tissue-specific stem cells know which type of cell to give rise to?
Gabriele Colozza, a postdoctoral researcher in Bon-Kyoung Koo’s lab at IMBA – now director of the Genome Engineering Center at the Institute for Basic Sciences in South Korea – decided to study this question using intestinal stem cells.
The intestines, a constant construction site
“In our intestines, cells are exposed to extreme conditions,” explains Colozza. Mechanical wear, but also digestive enzymes and pH variations affect intestinal cells. In turn, stem cells in the intestinal lining differentiate to form new intestinal cells.
“Damaged cells need to be replaced, but it is a delicate balance between renewing stem cells and differentiating into other cell types: uncontrolled proliferation of stem cells can lead to the formation of tumors; On the other hand, if too many stem cells differentiate, the tissue will be depleted of stem cells and ultimately unable to self-renew.
This balance is delicately regulated by signaling pathways and feedback loops, which allow cells to communicate with each other. One important pathway is called Wnt. The Wnt pathway is known for its role in embryonic development and, if left uncontrolled, an overly active Wnt pathway can lead to excessive cell division and tumor formation.
Molecular partner identified
A well-known antagonist of Wnt signaling, which controls Wnt, is Rnf43, which was initially identified by Bon-Kyoung Koo. Prior to this study, Rnf43 was known to target the Frizzled Wnt receptor and mark it for degradation.
“We wanted to know how Rnf43 works, and also what, in turn, controls Rnf43 and helps it regulate Wnt signaling.” From previous research, scientists knew that Rnf43 alone was not enough to break down the Frizzled Wnt receptor, located in the plasma membrane.
“In our project, we used biochemical assays to identify proteins that interact with Rnf43.” A key partner of Rnf43 was found to be the Daam1 protein.
To understand how Daam1 regulates Rnf43 and affects the tissues in which it acts, Colozza turned to intestinal organoids.
“We found that Daam1 is necessary for Rnf43 to be active, therefore for Rnf43 to regulate Wnt signaling. Subsequent work in cells showed that Rnf43 requires Daam1 to move the Wnt receptor Frizzled into vesicles called endosomes. Since endosomes, Frizzled is transported to lysosomes where it is degraded, thereby attenuating Wnt signaling,” adds Colozza.
Intestinal organoids are three-dimensional cell cultures grown from adult intestinal stem cells, allowing researchers to mimic the intestinal lining.
For Colozza, the organoids presented an opportunity to understand how Rnf43 and Daam1 affect the delicate balance of stem cell renewal and differentiation in the intestine. “We found that when we remove Rnf43 or Daam1, the organoids transform into tumor-like structures. These tumor-like organoids continue to grow, even if we remove the growth factors they usually depend on, such as R -spondin.”
Activation of Paneth cell formation
When Colozza followed up this result on mouse tissue, the researchers were surprised.
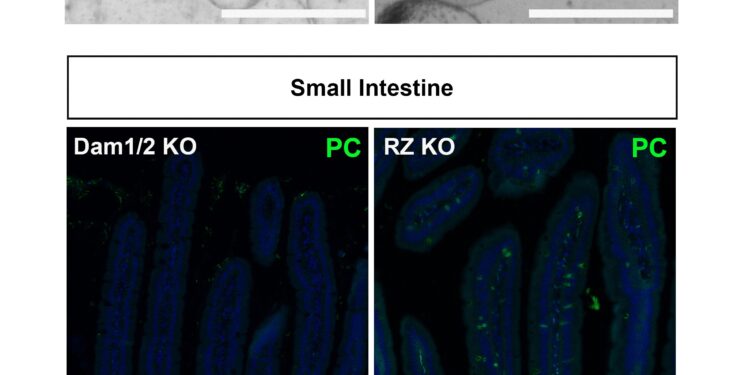
“When Rnf43 was missing, the intestines produced tumors, as expected. But when Daam1 was missing, no tumors developed. We were intrigued by this striking difference: how the loss of factors in the same pathway, which behave similarly in organoids, can it lead to such different results?
By looking closely at the intestines, Colozza found that intestines lacking Rnf43 were filled with a specific type of secretory cells, Paneth cells. In contrast, intestines lacking Daam1 contained no additional Paneth cells. Paneth cells secrete growth factors, such as Wnt, which stimulate cell division.
“Daam1 is required for the efficient formation of Paneth cells. When Daam1 is active, stem cells differentiate to form Paneth cells. When Daam1 is not active, stem cells differentiate into another cell type. ”
Tumors modify their niche to grow
This link between molecular findings and Paneth cells explains the puzzling difference between intestines and organoids.
“In organoid culture, we scientists provide growth factors, so inactivation of Rnf43 and Daam1 leads to tumor-like organoids. But in the intestine, no small scientist provides growth factors. Instead, Paneth cells provide growth factors, like Wnt., and create the right conditions for stem cells to survive and divide.
“When Paneth cells are lacking, such as when Daam1 is not active to drive cells to become Paneth cells, stem cells do not divide much. But when there are too many Paneth cells, such as In intestines lacking Rnf43, excessive growth factors may contribute to tumor formation.
The study by Colozza and colleagues is the first genetic evidence that Daam1, a member of the non-canonical Wnt pathway, is important for Paneth cell specification and directly involved in the development of this crucial secretory cell. The results also highlight the importance of the stem cell niche. “We show that tumor cells change their microenvironment and influence their supporting environment so that they can grow better.”
More information:
Gabriele Colozza et al, The differentiation of intestinal Paneth cells relies on an asymmetric regulation of Wnt signaling by Daam1/2, Scientists progress (2023). DOI: 10.1126/sciadv.adh9673. www.science.org/doi/10.1126/sciadv.adh9673
Provided by the Institute of Molecular Biotechnology of the Austrian Academy of Sciences
Quote: Decoding cell fate: key mechanism in stem cell change identified (November 24, 2023) retrieved November 25, 2023 from
This document is subject to copyright. Apart from fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.